Desoxycorticosterone
- CAS No.
- 64-85-7
- Chemical Name:
- Desoxycorticosterone
- Synonyms
- DOC;CORTEXONE;Corthormon;Deoxycortone;Desoxycorton;DOC【steroid】;DESOXYCORTONE;'REICHSTEIN Q';DEOXYCORTICOSTERONE;DESOXYCORTICOSTERONE
- CBNumber:
- CB4314147
- Molecular Formula:
- C21H30O3
- Molecular Weight:
- 330.46
- MDL Number:
- MFCD00003661
- MOL File:
- 64-85-7.mol
| Melting point | 138-144 °C |
|---|---|
| alpha | 184 º (c=1, C2H5OH) |
| Boiling point | 407.89°C (rough estimate) |
| Density | 1.0998 (rough estimate) |
| refractive index | 1.5192 (estimate) |
| Flash point | 9℃ |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 12.98±0.10(Predicted) |
| color | White to Pale Yellow |
| optical activity | +17822 (c 1.5, ethanol) |
| Water Solubility | slightly soluble |
| Merck | 13,2917 |
| BRN | 2062123 |
| LogP | 2.880 |
| CAS DataBase Reference | 64-85-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 40GP35YQ49 |
| ATC code | H02AA03 |
| NIST Chemistry Reference | Deoxycorticosterone(64-85-7) |
| EPA Substance Registry System | Deoxycorticosterone (64-85-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H351-H373 |
| Precautionary statements | P201-P308+P313 |
| Hazard Codes | Xn,T,F |
| Risk Statements | 40-48-39/23/24/25-23/24/25-11 |
| Safety Statements | 22-24/25-45-36/37-16-7 |
| RIDADR | UN 2811 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | HG0350000 |
Desoxycorticosterone price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | D6875 | 21-Hydroxyprogesterone ≥97% (HPLC) | 64-85-7 | 1g | $386 | 2024-03-01 | Buy |
| Sigma-Aldrich | D-105 | 11-Deoxycorticosterone solution 100?μg/mL in methanol, ampule of 1?mL, certified reference material, Cerilliant? | 64-85-7 | 1mL | $128 | 2024-03-01 | Buy |
| Cayman Chemical | 22916 | 11-deoxy Corticosterone ≥95% | 64-85-7 | 25mg | $55 | 2024-03-01 | Buy |
| Cayman Chemical | 22916 | 11-deoxy Corticosterone ≥95% | 64-85-7 | 50mg | $101 | 2024-03-01 | Buy |
| Sigma-Aldrich | D6875 | 21-Hydroxyprogesterone ≥97% (HPLC) | 64-85-7 | 500mg | $221 | 2024-03-01 | Buy |
Desoxycorticosterone Chemical Properties,Uses,Production
Chemical Properties
white to creamy-white crystalline powder
Uses
Antiinflammatory;Corticoide
Uses
A mineralocorticoid that occurs in adrenal cortex. It acts as a precursor to Aldosterone (A514700).
Definition
ChEBI: A mineralocorticoid that is progesterone substituted at position 21 by a hydroxy group.
General Description
11-Deoxycorticosterone is a steroid hormone produced in the adrenal glands that acts as a precursor for the hormone aldosterone. Levels of 11-deoxycorticosterone are measured by LC-MS/MS to aid in diagnosing disorders of steroid synthesis, such as 11-hydroxylase deficiency and glucocorticoid responsive hyperaldosteronism. This Certified Spiking Solution? is suitable for use as starting material inlinearity standards, calibrators, and controls for numerous LC-MS/MS applications in endocrinology, clinical chemistry, and neonatal screening.
Hazard
Toxic.
Mechanism of action
Desoxycorticosterone causes an increase in reabsorption of sodium ions and excretion of potassium ions from the renal tubules, which leads to increased tissue hydrophilicity. This facilitates an elevated volume of plasma and increased arterial pressure. Muscle tonicity and work capability are increased. It is used for an insufficiency of function of the adrenal cortex, myasthenia, asthenia, adynamia, and overall muscle weakness.
Synthesis
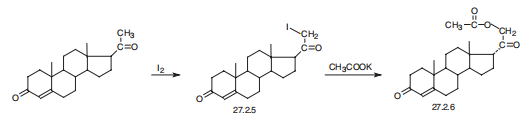
Desoxycorticosterone, 21-hydroxypregn-4-en-3,20-dione acetate (27.2.6), is synthesized in a number of ways, the easiest of which being iodination of progesterone at C21 in the methyl group, and subsequent reaction of the resulting iodo-derivative 27.2.5 with potassium acetate, which leads to formation of the desired desoxycorticosterone in the form of the acetate (27.2.6) .
Purification Methods
Crystallise 11-deoxycorticosterone from diethyl ether. [Schindler et al. Helv Chim Acta 24 360 1941, Steiger & Reichstein Helv Chim Acta 20 1164 1937.]
Desoxycorticosterone Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32165 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | info@fortunachem.com | China | 5978 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| China National Standard Pharmaceutical Corporation Limited | +8615391658522 | overseasales@yongstandards.com | China | 11922 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +86-15271838296; +8615271838296 | kyra@quanjinci.com | China | 1512 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33338 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57505 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | cooperation@kean-chem.com | China | 40066 | 58 |
View Lastest Price from Desoxycorticosterone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Desoxycorticosterone
64-85-7
|
US $0.00-0.00 / mg | 1mg | 98% | 10g/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-19 | Deoxycorticosterone
64-85-7
|
US $39.00-0.00 / mg | 97.91% | 10g | TargetMol Chemicals Inc. |
-

- Desoxycorticosterone
64-85-7
- US $0.00-0.00 / mg
- 98%
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Deoxycorticosterone
64-85-7
- US $39.00-0.00 / mg
- 97.91%
- TargetMol Chemicals Inc.







