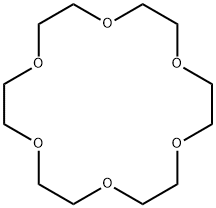
18-Crown-6
- CAS No.
- 17455-13-9
- Chemical Name:
- 18-Crown-6
- Synonyms
- 1,4,7,10,13,16-HEXAOXACYCLOOCTADECANE;8-Crown-6;HEXAOXACYCLOOCTADECANE;18-CROWN 6-ETHER;Ethylene oxide cyclic hexamer;18-Crown ether-6;18-CROWN-6;NSC 159836;18-Crown-6 99%;18-Crown-6,99%
- CBNumber:
- CB4445218
- Molecular Formula:
- C12H24O6
- Molecular Weight:
- 264.32
- MDL Number:
- MFCD00005113
- MOL File:
- 17455-13-9.mol
- MSDS File:
- SDS
| Melting point | 42-45 °C(lit.) |
|---|---|
| Boiling point | 116°C 0,2mm |
| Density | 1,175 g/cm3 |
| refractive index | 1.4580 (estimate) |
| Flash point | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Slightly), Methanol (Very Slightly) |
| form | Crystals or Crystalline Mass or Liquid |
| color | White or clear colorless |
| Water Solubility | SOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,2602 |
| BRN | 1619616 |
| Stability | Stable. Incompatible with strong acids, strong oxidizing agents. |
| InChIKey | XEZNGIUYQVAUSS-UHFFFAOYSA-N |
| Indirect Additives used in Food Contact Substances | 1,4,7,10,13,16-HEXAOXACYCLOOCTADECANE |
| CAS DataBase Reference | 17455-13-9(CAS DataBase Reference) |
| FDA UNII | 63J177NC5B |
| NIST Chemistry Reference | 1,4,7,10,13,16-Hexaoxacyclooctadecane(17455-13-9) |
| EPA Substance Registry System | 1,4,7,10,13,16-Hexaoxacyclooctadecane (17455-13-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P501 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-36/37/38-36-20/22-20/21/22 | |||||||||
| Safety Statements | 26-36-39 | |||||||||
| RIDADR | 2811 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | MP4500000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29329995 | |||||||||
| Toxicity | LD50 orally in Rabbit: 525 mg/kg LD50 dermal Rabbit 3888 mg/kg | |||||||||
| NFPA 704 |
|
18-Crown-6 price More Price(61)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.11684 | Crown ether/18-Crown-6 for synthesis | 17455-13-9 | 2g | $26.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.11684 | Crown ether/18-Crown-6 for synthesis | 17455-13-9 | 5G | $33.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.11684 | Crown ether/18-Crown-6 for synthesis | 17455-13-9 | 25g | $153 | 2024-03-01 | Buy |
| Sigma-Aldrich | 28125 | 18-Crown-6 purum, ≥99.0% (GC) | 17455-13-9 | 5g | $46.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 186651 | 18-Crown-6 99% | 17455-13-9 | 5G | $113 | 2024-03-01 | Buy |
18-Crown-6 Chemical Properties,Uses,Production
Chemical Properties
slightly yellow solid
Uses
18-Crown-6 may be used to catalyze the N-alkylation of heterocyclic compounds and allylation of functionalized aldehydes.
Uses
A useful phase transfer catalyst.
Uses
18-Crown-6 is used as an efficient phase transfer catalyst and as a complexing agent with a variety of small cation. It is involved in the synthesis of diaryl ethers, diaryl thioethers, and diarylamines mediated by potassium fluoride-alumina and 18-crown-6. It facilitates the solubility of potassium permanganate in benzene, which is used for oxidizing the organic compounds. It is used to accelerate various substitution reactions as well as enhances the power of nucleophiles such as potassium acetate. It is utilized in the alkylation reactions in the presence of potassium carbonate, N-alkylation of glutarimide and succinimide with dimethylcarbonate. The complex formed by its reaction with potassium cyanide acts as a catalyst in the cyanosilylation of aldehydes, ketones and quinines with trimethylsilyl cyanide (TMSCN).
Definition
ChEBI: 18-crown-6 is a crown ether that is cyclooctadecane in which the carbon atoms at positions 1, 4, 7, 10, 13 and 16 have been replaced by oxygen atoms. It has a role as a phase-transfer catalyst. It is a crown ether and a saturated organic heteromonocyclic parent.
Synthesis Reference(s)
The Journal of Organic Chemistry, 39, p. 2445, 1974 DOI: 10.1021/jo00930a037
General Description
18-Crown-6 is the simplest crown ether that can be prepared by reacting triethylene glycol with triethylene glycol dichloride in the presence of potassium hydroxide as a base. 18-Crown-6 can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst. It can also be used as a metal complexing agent to prepare a variety of molecular complexes.
Purification Methods
Recrystallise it from acetonitrile and dry it in a vacuum. Purify it also by precipitating the 18-crown-6/nitromethane 1:2 complex with Et2O/nitromethane (10:1 mixture). The complex is decomposed in vacuum whereby 18-crown-6 distils off under the reduced pressure. [Beilstein 19/12 V 601.]
18-Crown-6 Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shandong Believe Chemical PTE.LTD | +86-18553607090 +8618553607090 | 1093908296@qq.com | China | 1667 | 58 |
| weifang runzhong fine chemical co., ltd | 536-7868377 +8618653686003 | sq@runzhongchem.com | China | 187 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12839 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5892 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | bess@weibangbio.com | China | 18153 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20286 | 58 |
| Apeloa production Co.,Limited | +8619933239880 | admin@apcl.com.cn | China | 852 | 58 |
Related articles
- 18-Crown-6: Applications in Coordinated Metal Halides and Effect on Oxytocin Stability
- 18-Crown-6 enhances luminescence and nonlinear optical effects in metal halide complexes, and impacts oxytocin stability, hold....
- Oct 25,2024
- The Synthesis method and Toxicity of 18-Crown-6
- The compound known as 18-crown-6 is one of the simplest and most valuable of the macrocyclic polyethers.
- May 10,2024
- 18-Crown-6 coordination compounds: synthesis and properties
- 18-Crown-6 coordination compounds display intriguing properties in terms of photoluminescence and nonlinear optics.
- Jul 3,2023
View Lastest Price from 18-Crown-6 manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-27 | 18-Crown-6
17455-13-9
|
US $0.00 / KG | 1KG | 99%min | 30tons/month | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-19 | 18-Crown-6
17455-13-9
|
US $1.00 / g | 1g | 99% | 10000 | Apeloa production Co.,Limited | |
 |
2024-11-01 | 18-crown - 6 ether
17455-13-9
|
US $0.00 / PCS | 1PCS | 99% | 10 mt | Hebei Weibang Biotechnology Co., Ltd |
-

- 18-Crown-6
17455-13-9
- US $0.00 / KG
- 99%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- 18-Crown-6
17455-13-9
- US $1.00 / g
- 99%
- Apeloa production Co.,Limited
-

- 18-crown - 6 ether
17455-13-9
- US $0.00 / PCS
- 99%
- Hebei Weibang Biotechnology Co., Ltd
17455-13-9(18-Crown-6 )Related Search:
1of4