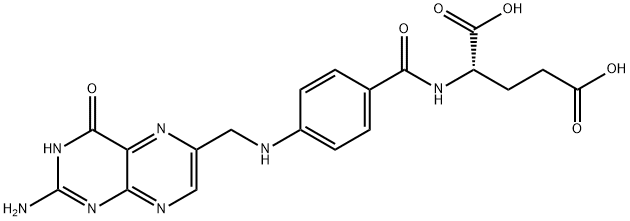
Folic acid
- CAS No.
- 59-30-3
- Chemical Name:
- Folic acid
- Synonyms
- PGA;Folate;Vitamin B;folic;Vitamin B11;PTEROYLMONOGLUTAMIC ACID;PTEROYLGLUTAMIC ACID;FOLIC ACID HYDRATE;PTEGLU;FOLACIN
- CBNumber:
- CB5100903
- Molecular Formula:
- C19H19N7O6
- Molecular Weight:
- 441.4
- MDL Number:
- MFCD00079305
- MOL File:
- 59-30-3.mol
- MSDS File:
- SDS
| Melting point | 250 °C |
|---|---|
| alpha | 20 º (c=1, 0.1N NaOH) |
| Boiling point | 552.35°C (rough estimate) |
| Density | 1.4704 (rough estimate) |
| refractive index | 1.6800 (estimate) |
| storage temp. | 2-8°C |
| solubility | boiling water: soluble1% |
| form | Crystalline Powder |
| pka | pKa 2.5 (Uncertain) |
| color | Yellow to orange |
| Odor | Odorless |
| PH Range | 4 |
| Water Solubility | 1.6 mg/L (25 ºC) |
| Merck | 14,4221 |
| BRN | 100781 |
| BCS Class | 3 |
| Stability | Stable. Incompatible with heavy metal ions, strong oxidizing agents, strong reducing agents. Solutions may be light and heat sensitive. |
| InChIKey | OVBPIULPVIDEAO-LBPRGKRZSA-N |
| LogP | -0.990 (est) |
| FDA 21 CFR | 172.345; 102.23; 104.20; 310.545 |
| Substances Added to Food (formerly EAFUS) | FOLIC ACID |
| CAS DataBase Reference | 59-30-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 935E97BOY8 |
| NCI Dictionary of Cancer Terms | folate; folic acid |
| NCI Drug Dictionary | folic acid |
| ATC code | B03BB01,B03BB51,V04CX02 |
| NIST Chemistry Reference | Folic acid(59-30-3) |
| EPA Substance Registry System | L-Glutamic acid, N-[4-[[(2-amino-3,4-dihydro-4-oxo-6-pteridinyl)methyl]amino]benzoyl]- (59-30-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Risk Statements | 33-62-68 |
| Safety Statements | 24/25 |
| WGK Germany | 1 |
| RTECS | LP5425000 |
| F | 8 |
| TSCA | Yes |
| HS Code | 29362900 |
| Hazardous Substances Data | 59-30-3(Hazardous Substances Data) |
| Toxicity | A water soluble vitamin required in the diet of mammals. Sulfonamide drugs are selectively toxic to bacteria because they inhibit the incorporation of p-aminobenzoic acid into folic acid, a biosynthetic process in bacteria. Folic acid deficiency adversely affects prenatal development in humans. Dietary supplementation with folic acid dramatically reduces the incidence of neural tube defects in humans. Folic acid deficiency may also contribute to the causes of megaloblastic macrocytic anemia and a consequence of this is that this disease can be induced, as a side effect, when methotrexate, a folic acid antagonist, is used in cancer chemotherapy |
Folic acid price More Price(55)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | F7876 | Folic acid ≥97% | 59-30-3 | 1g | $23.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1286005 | Folic acid United States Pharmacopeia (USP) Reference Standard | 59-30-3 | 500mg | $447 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP937 | Folic acid British Pharmacopoeia (BP) Reference Standard | 59-30-3 | 150MG | $266 | 2023-06-20 | Buy |
| TCI Chemical | F0043 | Folic Acid Hydrate >98.0%(HPLC)(T) | 59-30-3 | 25g | $65 | 2024-03-01 | Buy |
| Alfa Aesar | J60833 | Folic acid, crystalline | 59-30-3 | 25g | $80.2 | 2024-03-01 | Buy |
Folic acid Chemical Properties,Uses,Production
Description
Folic acid (folate,59-30-3) is a kind of B-vitamin which is mainly present in the liver and kidney. It has various kinds of pharmacological and physiological effects. It is involved in amino acid metabolism, purine and pyrimidine synthesis, and is also essential for hematopoiesis and red blood cell generation. In women pregnancy, folate can effectively prevent neural tube defects in the baby. It plays important role in fertility through contributing to spermatogenesis. It can also reduce the incidence of heart disease, stroke and cancer.
Folate deficiency may lead to various kinds of diseases including glossitis, diarrhea, depression, confusion, anemia, and fetal neural tube defectsand brain defects (during pregnancy). Other symptoms may include fatigue, gray hair, mouth sores, poor growth, and swollen tongue.
Description
Folic acid is an essential B vitamin. It is converted to folate in vivo, which is a necessary cofactor for a variety of biological processes, including nucleotide synthesis and, thus, DNA synthesis and repair, among others. A deficiency in dietary folic acid can lead to a range of developmental and cognitive disorders, most prominently neural tube defects and congenital heart defects.
Description
In the 1930s, brewer’s yeast was found to prevent anemia. Folic acid was later discovered to be the nutrient responsible for this effect and was purified in 1941 by Mitchell and coworkers (Mitchell et al., 1941). Folate is needed for biosynthesis of purines and thymidine for DNA and RNA synthesis in all cells. It is also involved in metabolism of some of the amino acids needed for protein synthesis, especially the conversions of serine to glycine and homocysteine to methionine. This makes the nutrient especially important during periods of rapid cell division, such as in pregnancy. Folate is also involved in transfer of one-carbon groups for methylation reactions. The role of folate in cell division was capitalized upon in the synthesis of aminopterin, a folate antagonist, which was one of the first anticancer drugs produced.
Chemical Properties
orange to yellow crystalline powder
Physical properties
The folates include a large number of chemically related species, each differing
with respect to the various substituents possible at three sites on the pteroylglutamic
acid basic structure. More than 170 different folates are theoretically possible. Not
all of these occur in nature; but it has been estimated that as many as 100 different
forms are found in animals. The folates from most natural sources usually have a
single carbon unit at N-5 and/or N-10; these forms participate in the metabolism of
the single-carbon pool.
Folic acid (pteroylmonoglutamic acid) is an orange-yellow crystalline substance
that is soluble in water but insoluble in ethanol or less polar organic solvents. It is
unstable to light, to acidic or alkaline conditions, to reducing agents, and, except in
dry form, to heat. It is reduced in vivo enzymatically (or in vitro with a reductant
such as dithionite) first to 7,8-dihydrofolic acid (FH2) and then to FH4; both of these
compounds are unstable in aerobic environments and must be protected by the pres ence of an antioxidant (e.g., ascorbic acid, 2-mer
Two derivatives of folic acid, each having an amino group in the place of the
hydroxyl at C-4, are folate antagonists of biomedical use: aminopterin (4-aminofolic
acid) and methotrexate (4-amino-N10-methylfolic acid). Aminopterin is used as a
rodenticide; methotrexate is an antineoplastic agent.
Originator
Folvite, Lederle, US ,1946
Occurrence
Synthetic
Uses
folic acid is generally used as an emollient. In vitro and in vivo skin studies now indicate its capacity to aid in DnA synthesis and repair, promote cellular turnover, reduce wrinkles, and promote skin firmness. There is some indication that folic acid may also protect DnA from uV-induced damage. Folic acid is a member of the vitamin B complex and is naturally occurring in leafy greens.
Uses
hematopoietic vitamin
Uses
A vitamin needed to synthesize DNA, conduct DNA repair and methylate DNA, it also acts as a cofactor in biological reactions involving folate.
Uses
Folic Acid is a water-soluble b-complex vitamin that aids in the for- mation of red blood cells, prevents certain anemias, and is essential in normal metabolism. high-temperature processing affects its sta- bility. it is best stored at lower than room temperatures. it is also termed folacin. it is found in liver, nuts, and green vegetables.
Uses
Literature tends to indicate that B vitamins cannot pass through the layers of the skin and, therefore, are of no value in the skin surface. Current experiments demonstrate, however, that vitamin B2 acts as a chemical reaction accelerator, enhancing the performance of tyrosine derivatives in suntan-accelerating preparations.
Definition
ChEBI: An N-acyl-amino acid that is a form of the water-soluble vitamin B9. Its biologically active forms (tetrahydrofolate and others) are essential for nucleotide biosynthesis and homocysteine remethylation.
Manufacturing Process
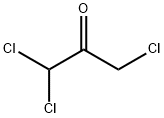
The following description is taken from US Patent 2,956,057.
100 grams of 1,3,3-trichloroacetone are heated on a boiling water bath and
95 grams of bromine are added thereto in drops while being stirred and the
stirring is continued for about 1 hour. The resulting reaction solution is
distilled under reduced pressure. 115 grams of 1-bromo-1,3,3-
trichloroacetone are obtained having a boiling point of 85° to 95°C/17 mm
(Hg).
For the preparation of the hydrate, 100 grams of water are added to 100
grams of 1bromo-1,3,3-trichloroacetone, which is agitated and cooled. A white
scaly crystal of hydrate of 1-bromo-1,3,3-trichloroacetone is obtained (100
grams), having a melting point of 52° to 53°C.
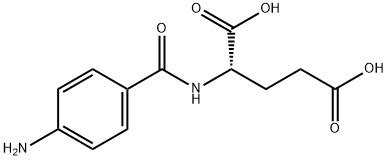
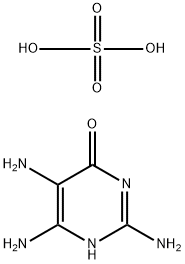
8.9 grams of 2,4,5-triamino-6-hydroxypyrimidine hydrochloride and 8 grams
of p-aminobenzoylglutamic acid are dissolved in 400 cc warm water, which is
cooled at 35° to 27°C and adjusted to pH 4 by using 20% caustic soda
solution. To this solution was simultaneously added dropwise a solution
obtained by dissolving 13.4 grams of 1-bromo-1,3,3-trichloroacetone hydrate
in 90 cc of 50% methanol and 24 grams of 35% aqueous sodium bisulfite
solution over a period of approximately 2 hours. During this period, in order
to maintain the pH value of the reaction solution at 4 to 5, 20% caustic soda
solution is added from time to time. The precipitate, formed by stirring for 5
hours after dropping was finished, is filtered, and the filtrated precipitate is
refined; 5.6 grams of pure pteroylglutamic acid is obtained.
Therapeutic Function
Treatment of B vitamin (folacin) deficiency
General Description
Odorless orange-yellow needles or platelets. Darkens and chars from approximately 482°F.
Air & Water Reactions
Insoluble in water. Aqueous solutions have pHs of 4.0-4.8.
Reactivity Profile
Acid solutions of Folic acid are sensitive to heat, but towards neutrality, stability progressively increases. Solutions are inactivated by ultraviolet light and alkaline solutions are sensitive to oxidation. Folic acid is also inactivated by light. Folic acid is incompatible with oxidizing agents, reducing agents and heavy metal ions.
Fire Hazard
Flash point data for Folic acid are not available; however, Folic acid is probably combustible.
Biochem/physiol Actions
A nutritional delivery form of folate. Folic acid and its derivatives are essential mediators of one-carbon metabolism within cells.
Clinical Use
Folate-deficient megaloblastic anaemia
Supplement in HD patients
Safety Profile
Poison by intraperitoneal and intravenous routes. Experimental teratogenic effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Veterinary Drugs and Treatments
Folic acid is used to treat folic acid deficiency in dogs, cats, and horses (theoretically in other animal species as well) often due to small intestinal disease. Cats with exocrine pancreatic insufficiency appear to be most at risk for folate and cobalamin deficiencies secondary to malabsorption of folic acid in the diet. Dogs with exocrine pancreatic insufficiency often are noted to have increased folate levels secondary to overgrowths of folate-synthesizing bacteria in the proximal small intestine. Chronic administration of dihydrofolate reductase inhibiting drugs such as pyrimethamine, ormetoprim or trimethoprim can potentially lead to reduced activated folic acid (tetrahydrofolic acid); folic acid supplementation is sometimes prescribed in an attempt to alleviate this situation.
Drug interactions
Potentially hazardous interactions with other drugs
Antiepileptics: reduces phenytoin, primidone and
phenobarbital levels.
Cytotoxics: avoid with raltitrexed.
Metabolism
Folic acid given therapeutically enters the portal circulation largely unchanged, since it is a poor substrate for reduction by dihydrofolate reductase. It is converted to the metabolically active form 5-methyltetrahydrofolate in the plasma and liver. Folate undergoes enterohepatic circulation. Folate metabolites are eliminated in the urine and folate in excess of body requirements is excreted unchanged in the urine.
Purification Methods
If paper chromatography indicates impurities, then recrystallise it from hot H2O or from dilute acid [Walker et al. J Am Chem Soc 70 19 1948]. Impurities may be removed by repeated extraction with n-BuOH of a neutral aqueous solution of folic acid (by suspending in H2O and adding N NaOH dropwise till the solid dissolves, then adjusting the pH to ~7.0-7.5) followed by precipitation with acid, filtration, or better collected by centrifugation and recrystallised form hot H2O. [Blakley Biochem J 65 331 1975, Kalifa et al. Helv Chim Acta 6 1 2739 1978.] Chromatography on cellulose followed by filtration through charcoal has also been used to obtain pure acid. [Sakami & Knowles Science 129 274 1959.] UV: max 247 and 296nm ( 12,800 and 18,700) in H2O pH 1.0; 282 and 346nm ( 27.600 and 7,200) in H2O pH 7.0; 256, 284 and 366nm ( 24600, 24,500 and 86,00) in H2O pH 13 [Rabinowitz in The Enzymes (Boyer et al. Eds), 2 185 1960]. [Beilstein 26 III/IV 3944.]
Folic acid Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TAIZHOU YUXIN BIOTECHNOLOGY CO,.LTD | +86-576-88902229;+86-0576-88902229 +8613968687450 | yuxin@yuxchem.com | China | 167 | 58 |
| XI AN SGONEK BIOLOGICAL TECHNOLOGY CO., LTD | 15202951039 | jason@sgonekbio.com | China | 268 | 58 |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 | info@deshangchem.com | China | 662 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 | admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 12272 | 58 |
| HUARONG(GUANGDONG) PHARMACEUTICAL CO.,LTD | +86-19925801629 +86-19925801629 | eric@huarongpharma.com | China | 171 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3882 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12835 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5889 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | bess@weibangbio.com | China | 18151 | 58 |
Related articles
- Folate synthesis
- The synthetic form of folate is folic acid. It's in an essential component of prenatal vitamins and is in many fortified foods....
- Jul 25,2024
- Efficacy and effects of folic acid supplements on the human body
- Folic acid is the active form of vitamin B9 and occurs naturally in many foods. Folic acid helps the body make healthy red blo....
- Jan 12,2024
- Aplications and Side effects of Folic acid
- Folic acid is a type of B vitamin that is normally found in foods such as dried beans, peas, lentils, oranges, whole-wheat pro....
- Oct 12,2019
View Lastest Price from Folic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-20 | Vitamin B9 (folic acid)
59-30-3
|
US $0.00 / kg | 25kg | 99.0% | 10tons | Henan Suikang Pharmaceutical Co.,Ltd. | |
 |
2024-12-20 | Folic acid
59-30-3
|
US $100.00-75.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-12-20 | Folic acid
59-30-3
|
US $0.00 / kg | 25kg | BP/USP Feed grade | 100MT | HUARONG(GUANGDONG) PHARMACEUTICAL CO.,LTD |
-

- Vitamin B9 (folic acid)
59-30-3
- US $0.00 / kg
- 99.0%
- Henan Suikang Pharmaceutical Co.,Ltd.
-

- Folic acid
59-30-3
- US $100.00-75.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Folic acid
59-30-3
- US $0.00 / kg
- BP/USP Feed grade
- HUARONG(GUANGDONG) PHARMACEUTICAL CO.,LTD





