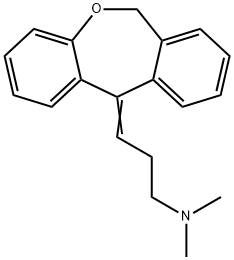
DOXEPIN
- CAS No.
- 1668-19-5
- Chemical Name:
- DOXEPIN
- Synonyms
- Aponal;p3693a;Curatin;Docepin;P-3693A;Quitaxon;Doxepine;NSC-108160;Doxepin-13C D3;DOXEPIN USP/EP/BP
- CBNumber:
- CB5113073
- Molecular Formula:
- C19H21NO
- Molecular Weight:
- 279.38
- MDL Number:
- MFCD00865448
- MOL File:
- 1668-19-5.mol
- MSDS File:
- SDS
| Melting point | 187-189°C |
|---|---|
| Boiling point | bp0.03 154-157°; bp0.2 260-270° |
| Density | 1.0594 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| pka | 9.40±0.28(Predicted) |
| Water Solubility | 31.57mg/L(25 ºC) |
| FDA UNII | 5ASJ6HUZ7D |
| EPA Substance Registry System | Doxepin (1668-19-5) |
SAFETY
Risk and Safety Statements
| Hazard Codes | T |
|---|---|
| Risk Statements | 25 |
| Safety Statements | 36/37/39-45 |
| Hazardous Substances Data | 1668-19-5(Hazardous Substances Data) |
| Toxicity | LD50 in mice, rats (mg/kg): 26, 16 i.v.; 79, 182 i.p.; 135, 147 orally (Ribbentrop, Schaumann) |
DOXEPIN price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Medical Isotopes, Inc. | 9811 | Doxepin hydrochloride | 1668-19-5 | 1g | $90 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 9811 | Doxepin hydrochloride | 1668-19-5 | 5G | $290 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0006489 | DOXEPIN 95.00% | 1668-19-5 | 1G | $1004.85 | 2021-12-16 | Buy |
| Crysdot | CD11240881 | 3-(Dibenzo[b,e]oxepin-11(6H)-ylidene)-N,N-dimethylpropan-1-amine 95+% | 1668-19-5 | 1g | $470 | 2021-12-16 | Buy |
DOXEPIN Chemical Properties,Uses,Production
Originator
Sinequan,Pfizer,US,1969
Uses
Used clinically to treat anxiety and depression. Antidepressant
Definition
ChEBI: A dibenzooxepine that is 6,11-dihydrodibenzo[b,e]oxepine substituted by a 3-(dimethylamino)propylidene group at position 11. It is used as an antidepressant drug.
Manufacturing Process
(A) Preparation of 3-bromopropyltriphenylphosphonium bromide:
Triphenylphosphine, 1.0 kg, and 770 grams of 1,3-dibromopropane are
dissolved in 2.0 liters of xylene and the solution is stirred under a nitrogen
atmosphere at 130°C. After 20 hours the mixture is cooled, and the crystalline
product, which precipitates, is collected and washed with 20 liters of benzene.
After drying in vacuo the product weighs 1,578 grams, MP 229°-230°C;titration for bromide ion: Found, 17.1%; calculated, 17.2%.
(B) Preparation of 3-dimethylaminopropyltriphenylphosphonium bromide
hydrobromide: A solution of 595 grams of anhydrous dimethylamine and
1,358 grams of 3-bromopropyl-triphenylphosphonium bromide in 4 liters of
ethanol is warmed to 70°C until solution is complete and the solution then is
allowed to stand at room temperature for 20 hours. Volatile components are
removed by distillation in a vacuum and the residue is suspended in 2.0 liters
of ethanol and is redistilled to remove excess amine. The residue is dissolved
in 3.0 liters of warm ethanol and gaseous hydrogen bromide is passed into
the solution until the mixture is acidic. After filtration the solution is
concentrated to a volume of 3.0 liters, is cooled, whereupon the product
precipitates, and the precipitate is collected; it weighs 1,265 grams, MP 274-
281°C. Recrystallization from ethanol raises the MP to 280.5°-282.5°C.
Bromide ion titration: Found, 31.2%; calculated 31.3%.
(C) Preparation of doxepin: 1,530 grams of the product from step (B) is
suspended in 4.5 liters dry tetrahydrofuran and 6.0 mols of butyl lithium in
heptane is added during 1 hour. After an additional 30 minutes, 483 grams of
6,11-dihydrodibenz-(b,e)oxepin-11-one, prepared as described in Belgian
Patent 641,498, is added to the deep red solution and the reaction was
maintained at reflux for 10 hours. Water, 500 ml, is added at room
temperature and the solvent is removed in vacuo. The crude residue is treated
with 10% hydrochloric acid until acidic (pH 2) and then 1.5 liters benzene is
added. After stirring, the mixture separates into 3 phases (an insoluble
hydrochloride salt product phase, an aqueous phase and an organic phase).
The benzene layer is removed by decantation and the remaining mixture is
rendered basic with 10% sodium hydroxide solution and is extracted with
three 1,500 ml portions of benzene. The benzene extracts are washed, then
dried with anhydrous sodium sulfate and concentrated in a vacuum leaving a
residue of 1,530 grams, gas and thin layer chromatography analysis show this
to be a cis/trans mixture (approx. 4:l) of 11-dimethylaminopropylidene-6,11-
dihydrodibenz-(b,e)oxepin (90% yield). This mixture has substantially more
activity pharmacologically than the cis/trans mixture obtained by the Grignard
route disclosed in the Belgian Patent 641,498. This base is then converted to
the hydrochloride with HCl.
brand name
Apo-doxepin;Co dox;Deptran;Doksapan;Dolat;Doxal;Doxedyn;Doxepin hcl;Gilex;Novo-doxepin;Novoxapin;Sinequan;Sinquan concentrate;Sinquane;Tolllluan;Triadapin;Zonalon.
Therapeutic Function
Tranquilizer
World Health Organization (WHO)
Doxepin, a tricyclic antidepressant was introduced in 1964 for the management of endogenous depression. Much of the adverse effects are caused by its antimuscarinic actions. These include dry mouth, cardiac arrhythmias, central nervous system disturbances, blood disorders and risk of suicide. The risk of suicide and dangers related to overdosage led the Norwegian Medicines Control Authority to put the higher strength formulation under prescribing restriction in 1992. The risk of death following overdosage is apparently higher for products containing tricyclic compounds as compared with nontricyclic products.
Biological Activity
Highly potent H 1 histamine receptor antagonist (K d = 310 pM) and tricyclic antidepressant. Also binds to the H 4 histamine receptor (pK i = 6.79).
Contact allergens
This benzoxepin tricylcic drug has antidepressant, anticholinergic, antiitching, and antihistamine properties. After oral use, it has been developed as a topical antiitching agent. Allergic contact dermatitis is not infrequent.
DOXEPIN Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63687 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29808 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 22854 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49734 | 58 |
| Aladdin Scientific | tp@aladdinsci.com | United States | 52924 | 58 | |
| TargetMol Chemicals Inc. | +8613564774135 | zijue.cai@tsbiochem.com | United States | 19881 | 58 |
| 821-50328103-801 18930552037 | 3bsc@sina.com | China | 15839 | 69 | |
| LGM Pharma | 1-(800)-881-8210 | inquiries@lgmpharma.com | United States | 2123 | 70 |
| BOC Sciences | 1-631-485-4226; 16314854226 | info@bocsci.com | United States | 12952 | 65 |
| Beijing HuaMeiHuLiBiological Chemical | 010-56205725 | waley188@sohu.com | China | 12335 | 58 |
| Supplier | Advantage |
|---|---|
| Alchem Pharmtech,Inc. | 58 |
| career henan chemical co | 58 |
| Dideu Industries Group Limited | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | 58 |
| Aladdin Scientific | 58 |
| TargetMol Chemicals Inc. | 58 |
| 69 | |
| LGM Pharma | 70 |
| BOC Sciences | 65 |
| Beijing HuaMeiHuLiBiological Chemical | 58 |
View Lastest Price from DOXEPIN manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-28 | Doxepin
1668-19-5
|
US $1980.00-1520.00 / mg | 10g | TargetMol Chemicals Inc. | |||
 |
2021-07-27 | DOXEPIN USP/EP/BP
1668-19-5
|
US $1.10 / g | 1g | 99.9% | 100 Tons min | Dideu Industries Group Limited | |
 |
2020-01-10 | DOXEPIN
1668-19-5
|
US $7.00 / KG | 1KG | 99% | 100KG | Career Henan Chemical Co |
-

- Doxepin
1668-19-5
- US $1980.00-1520.00 / mg
- TargetMol Chemicals Inc.
-

- DOXEPIN USP/EP/BP
1668-19-5
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited
-

- DOXEPIN
1668-19-5
- US $7.00 / KG
- 99%
- Career Henan Chemical Co