Rucaparib
- CAS No.
- 283173-50-2
- Chemical Name:
- Rucaparib
- Synonyms
- Rucaparib Base;8-fluoro-5-(4-((methylamino)methyl)phenyl)-2,3,4,6-tetrahydro-1H-azepino[5,4,3-cd]indol-1-one;Rikapbu;AG-14447;AG014447;Rucaparib;AG 014447;PF-01367388;AG 014447;AG014447;Rucaparib impurity
- CBNumber:
- CB51475507
- Molecular Formula:
- C19H18FN3O
- Molecular Weight:
- 323.36
- MDL Number:
- MFCD11977252
- MOL File:
- 283173-50-2.mol
| Melting point | 187 - 189°C |
|---|---|
| Boiling point | 625.2±55.0 °C(Predicted) |
| Density | 1.281 |
| storage temp. | 2-8°C(protect from light) |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Yellow solid. |
| pka | 14.10±0.20(Predicted) |
| color | Pale Yellow to Yellow |
| FDA UNII | 8237F3U7EH |
| ATC code | L01XK03 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H361 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501 |
Rucaparib price More Price(38)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 15643 | Rucaparib ≥98% | 283173-50-2 | 1mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 15643 | Rucaparib ≥98% | 283173-50-2 | 5mg | $100 | 2024-03-01 | Buy |
| Cayman Chemical | 15643 | Rucaparib ≥98% | 283173-50-2 | 10mg | $153 | 2024-03-01 | Buy |
| Cayman Chemical | 15643 | Rucaparib ≥98% | 283173-50-2 | 25mg | $343 | 2024-03-01 | Buy |
| TRC | R701580 | Rucaparib | 283173-50-2 | 10mg | $110 | 2021-12-16 | Buy |
Rucaparib Chemical Properties,Uses,Production
Description
Rucaparib is a PARP inhibitor used as an anti-cancer agent. Rucaparib is a first-in-class pharmaceutical drug targeting the DNA repair enzyme poly-ADP ribose polymerase-1 (PARP-1). It was discovered as part of a collaboration between scientists working at the Northern Institute of Cancer Research and Medical School of Newcastle University and Agouron Pharmaceuticals in San Diego, California. It is being developed by Clovis Oncology.
Application
Rucaparib is used to help maintain the response to other treatments for certain types of ovarian cancer (cancer that begins in the female reproductive organs where eggs are formed), fallopian tube (tube that transports eggs released by the ovaries to the uterus), and primary peritoneal (layer of tissue that lines the abdomen) cancer It is also used to treat certain types of ovarian cancer, fallopian tube cancer, and primary peritoneal cancer in people with a specific gene who have not improved after treatment with at least two other therapies. Rucaparib is in a class of medications called poly (ADP-ribose) polymerase (PARP) inhibitors. It works by killing cancer cells.
Mechanism of action
Rucaparib inhibits "the contraction of isolated vascular smooth muscle, including that from the tumours of cancer patients. It also reduces the migration of some cancer and normal cells in culture." As a PARP inhibitor, rucaparib is expected to be more effective in the 9% of pancreatic cancers with a BRCA mutation (BRCA1 or BRCA2).
Description
Rucaparib was approved in the US as an oral treatment for advanced ovarian cancer. Development of rucaparib began with collaborations between Cancer Research UK and Agouron Pharmaceuticals (later acquired by Pfizer). Global development rights for rucaparib were ultimately granted to Clovis Oncology via a licensing agreement from Pfizer. To qualify for treatment with rucaparib monotherapy, patients must demonstrate deleterious breast cancer (BRCA) mutation (germline and/or somatic)- associated advanced ovarian cancer and also must have previously been treated with two or more chemotherapy regimens. Rucaparib functions as a small molecule poly(ADPribose) polymerase (PARP) inhibitor, which plays an important role in DNA repair. This newly approved drug displays nanomolar potency against PARP-1, -2, and -3 enzymes, which translates into improved efficacy over alternative therapies such as olaparib or niraparib. Furthermore, rucaparib is also known to cause vasodilation, which is thought to induce tumor perfusion and increased accumulation of the drug in cancer cells. Although rucaparib shows higher cytotoxicity in cancer cells with mutation of BRCA1/2 genes and other DNA repair genes, reduced tumor growth was observed in mouse xenograft models of human cancers with and without BRCA mutations.Rucaparib is also being pursued as a treatment for breast cancer and has displayed promising initial results in trials for pancreatic cancer.
Description
Poly(ADP-
Uses
Rucaparib is PARP1 inhibitor. It can be used in biological study of chemical screening to identify drugs that enhance or mitigate cellular responses to antibody-toxin fusion proteins using human B cell precursor leukemia cells and cervical adenocarcinoma cells.
Definition
ChEBI: Rucaparib is a member of the class of azepinoindoles that is 1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one carrying additional 4-[(methylamino)methyl]phenyl and fluoro substituents at positions 2 and 8 respectively. It is an inhibitor of poly (ADP-ribose) polymerase and is used (as the camsylate salt) as monotherapy for advanced ovarian cancer and deleterious germline or somatic BRCA mutation. It has a role as an EC 2.4.2.30 (NAD(+) ADP-ribosyltransferase) inhibitor and an antineoplastic agent. It is an azepinoindole, a member of caprolactams, an organofluorine compound and a secondary amino compound. It is a conjugate base of a rucaparib(1+).
Synthesis
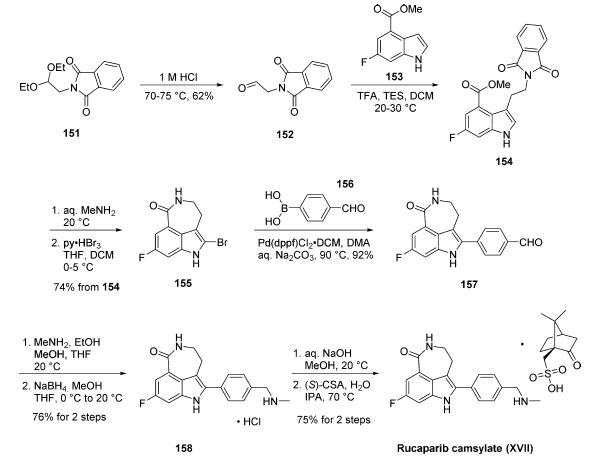
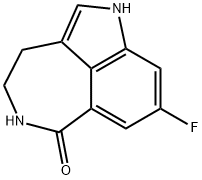
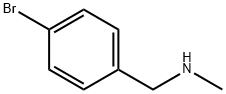
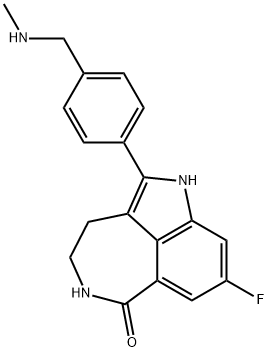
Synthesis of rucaparib camsylate begins from commercially available phthalimide acetal 151. Unveiling of the aldehyde via treatment with aqueous HCl and precipitation from toluene provided aldehyde 152 in 62% yield. To avoid polymerization, 152 was immediately subjected to 6-fluoro-1Hindole- 4-carboxylic acid methyl ester (153) under reductive conditions in the presence of acid to give rise to tryptamine derivative 154. After considerable research, optimal conditions for this transformation (triethylsilane in DCM/TFA) were found that were successful on up to 15.7 kg scale, enabling clean separation of the aldehyde reduction byproduct following crystallization. Conversion of the phthalimide within 154 to the corresponding amine using aqueous methylamine at room temperature was accompanied by an intramolecular cyclization reaction to secure the intermediate lactam as a solid in 89% isolated yield. This was followed by a high-yielding bromination reaction (83%) at the indole C-2 position employing pyridinium tribromide, providing access to indoloazepinone 155. After screening various catalysts for the coupling of bromide 155 and commercial boronic acid 156, Pd(dppf)Cl2?¤ DCM was found to reliably deliver the desired coupling product with reasonable rates of reaction. Thus, after development of an extensively optimized reaction protocol, Suzuki coupling of 155 and 4-formylphenylboronic acid (156) with Pd(dppf)Cl2?¤DCM and Na2CO3 in DMA at 90 ??C generated the desired 2-arylated indole 157 in high yield (92%) after trituration and reslurry with methanol. Conversion of aldehyde 157 to amine 158 necessitated a two-pot procedure designed to limit the formation of dimerization and aldehyde reduction products that generally arise under conventional onepot reductive amination conditions and have traditionally been problematic on scale. Toward this end, subjection of aldehyde 157 to a methylamine solution in EtOH/MeOH/THF and wash of the resulting reaction solids with methanol led to efficient isolation of pure imine intermediate, which could be immediately reduced with NaBH4 in THF/MeOH, providing hydrochloride salt 158 upon acidic workup in 76% over two steps. The two remaining steps for conversion to the drug involve a salt-swap, first breaking the HCl salt with NaOH in MeOH, then treatment with (S)-camphorsulfonic acid/IPA/ H2O at 70 ??C. Filtration and washing of the cake with water generated rucaparib camsylate (XVII) in 95% yield.
Rucaparib Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Zhejiang ZETian Fine Chemicals Co. LTD | +8618957127338 | stella@zetchem.com | China | 2136 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18751 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9087 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3009 | 60 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32957 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29880 | 58 |
| Jinan Shengqi pharmaceutical Co,Ltd | 86+18663751872 | christine@shengqipharm.com | CHINA | 491 | 58 |
Related articles
- Rucaparib: pharmacodynamics, pharmacokinetics and side effects
- Rucaparib, a potent PARP inhibitor, demonstrates strong anti-cancer activity but poses potential adverse effects.
- Nov 24,2023
Related Qustion
- Q:What Rucaparib Is Used For?
- A:Rucaparib is the generic name for the trade name drug Rubraca?. In some cases, health care professionals my use the trade name....
- Oct 29,2019
View Lastest Price from Rucaparib manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Rucaparib
283173-50-2
|
US $41.00-117.00 / mg | 99.89% | 10g | TargetMol Chemicals Inc. | ||
 |
2022-10-01 | Rucaparib
283173-50-2
|
US $0.00-0.00 / kg | 1kg | 98% | 1Ton | Henan Aochuang Chemical Co.,Ltd. | |
 |
2021-07-13 | Rucaparib
283173-50-2
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd |





