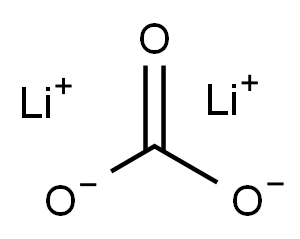
Lithium carbonate
- CAS No.
- 554-13-2
- Chemical Name:
- Lithium carbonate
- Synonyms
- Li2CO3;Eskalith;Carbolith;teralithe;carbolite;LITHIUM CARBONATE PURE;Lithium carbonate, TraceSelect;Limas;Phasal;Plenur
- CBNumber:
- CB5852228
- Molecular Formula:
- CLi2O3
- Molecular Weight:
- 73.89
- MDL Number:
- MFCD00011084
- MOL File:
- 554-13-2.mol
- MSDS File:
- SDS
| Melting point | 720 °C |
|---|---|
| Boiling point | 1342 °C(lit.) |
| Density | 2.11 g/mL at 25 °C |
| Flash point | 1310°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 13g/l |
| form | wire |
| pka | pKa 6.38 (Uncertain);10.25 (Uncertain) |
| Specific Gravity | 2.11 |
| color | White |
| PH | 10-11 (5g/l, H2O, 20℃) |
| Odor | odorless |
| Water Solubility | 13 g/L (20 ºC) |
| Merck | 14,5527 |
| Solubility Product Constant (Ksp) | pKsp: 1.6 |
| BRN | 3999191 |
| BCS Class | 1 |
| InChIKey | XGZVUEUWXADBQD-UHFFFAOYSA-L |
| LogP | -0.809 (est) |
| CAS DataBase Reference | 554-13-2(CAS DataBase Reference) |
| EWG's Food Scores | 3 |
| FDA UNII | 2BMD2GNA4V |
| Proposition 65 List | Lithium Carbonate |
| NCI Drug Dictionary | Eskalith |
| NIST Chemistry Reference | Lithium carbonate(554-13-2) |
| EPA Substance Registry System | Lithium carbonate (554-13-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H319 | |||||||||
| Precautionary statements | P264-P270-P280-P301+P312-P305+P351+P338-P337+P313 | |||||||||
| Hazard Codes | Xn,C,F | |||||||||
| Risk Statements | 36/38-41-36/37/38-22-36-34-20/21/22-15-14-11 | |||||||||
| Safety Statements | 8-43-45-37/39-26-36/37-24/25-36/37/39-16-7/8-3/7/9 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | OJ5800000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28369100 | |||||||||
| Toxicity | LD50 orally in rats: 0.71 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Lithium carbonate price More Price(102)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | NIST924A | Lithium carbonate NIST? SRM? 924a | 554-13-2 | 30g | $914 | 2024-03-01 | Buy |
| Sigma-Aldrich | 62470 | Lithium carbonate puriss. p.a., ACS reagent, reagent (for microscopy), ≥99.0% (T) | 554-13-2 | 100g | $49.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 62470 | Lithium carbonate puriss. p.a., ACS reagent, reagent (for microscopy), ≥99.0% (T) | 554-13-2 | 500g | $210 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1369000 | Lithium carbonate United States Pharmacopeia (USP) Reference Standard | 554-13-2 | 300mg | $436 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.70329 | Lithium ICP Standard traceable to SRM from NIST LiNO? in HNO? 2-3% 1000 mg/l Li Certipur? | 554-13-2 | 100mL | $102 | 2024-03-01 | Buy |
Lithium carbonate Chemical Properties,Uses,Production
Description
Lithium carbonate (molecular structure is Li2CO3, English name is lithium carbonate) as a colorless monoclinic crystal or white powder. Density is 2.11. Melting point is 618 ℃. Without deliquescence, it is stable in the air. Low solubility in water, the solubility decreases with increasing temperature. Solubility in cold water is greater than hot water. It is Soluble in dilute acid, insoluble in alcohol and acetone. Carbon dioxide is introduced into the aqueous suspension of lithium carbonate, lithium carbonate is converted to lithium acid carbonate and dissolved. If the solution of lithium acid carbonate is heated and then it releases carbon dioxide and precipitates lithium carbonate. The nature of the lithium carbonate may be used to remove impurities from lithium carbonate. Since lithium ion has a strong polarizability, thus thermal stability of lithium carbonate is worse than other alkali metal carbonate, when heated to above the melting point, it will decompose to produce carbon dioxide and lithium oxide.
Chemical Properties
Lithium carbonate is a white monoclinic crystalline solid. Typically for carbonates, lithium carbonate reacts with acids stronger than carbon dioxide or carbonic acid to yield the lithium salt of the acid and carbon dioxide. The reactions may be carried out in a solution, as an aqueous slurry, or, less effectively, with solid lithium carbonate.
Lithium carbonate exhibits a low water solubility for an alkali metal carbonate. The solubility decreases with increasing temperature. It is not hygroscopic and is generally stable when exposed to the atmosphere. In fact, it is the normal end compound encountered when many basic lithium compounds are exposed to the atmosphere. Lithium carbonate may be dissolved in water by conversion to the hydrogen carbonate. Releasing carbon dioxide by heating the solution of lithium hydrogen carbonate causes reprecipitation of the lithium carbonate.
Uses
The lithium carbonate industry is a global high monopoly industry, the current production capacity is mainly concentrated in three foreign manufacturers of SQM, FMC, Chemetall and so on.
Industrial lithium carbonate is used in the manufacture of other lithium salts, such as lithium chloride and lithium bromide and so on. It also acts as lithium oxide materials in enamel, glass, pottery and porcelain enamel, and it is also added to the electrolytic cell for electrolysis of aluminum to increase the current efficiency and reduce the internal resistance of the cell and the bath temperature. In medicine, it is mainly used for the treatment of mania, can improve their emotional disorders for schizophrenia. It has the effect of elevating peripheral leukocytes; can be used for synthetic rubber, dyes, semiconductor and military defense industry and so on; for the production of lithium tantalate, lithium niobate and other acoustic grade single crystal, optical grade monocrystalline etc; for preparation of the acoustic grade single crystal.
Battery grade lithium carbonate is mainly used for the preparation of lithium cobalt oxide, lithium manganese oxide, ternary materials, lithium iron phosphate and other lithium ion battery cathode materials; used in a matrix modifier; as aneuroprotective effect of lithium carbonate in amyotrophic lateral sclerosis.
Toxicity
Lithium carbonate has a significant stimulating effect, firstly has damage on the gastrointestinal tract, kidney and central nervous system. Toxicity order of lithium compounds is Li <LiCl <Li2CO3, maximum allowable concentration: Lithium condensation and fragmentation aerosol were 0.05 mg/m3 and 0.5 mg/m3.
Wear rubber gloves and protective masks when working, in order to protect the respiratory organs against dust.
Preparation
Lithium carbonate is obtained as an intermediate product in recovery of lithium metal from its ore, spodumene (See Lithium). It is prepared by mixing a hot and concentrated solution of sodium carbonate with lithium chloride or sulfate solution.
Li2SO4+ Na2CO3→Li2CO3+ Na2SO4
Reactions
Lithium carbonate reacts with dilute acids, liberating carbon dioxide:
Li2CO3+ HCl →LiCl + CO2+ H2O
Thermal decompostion yields lithium oxide and carbon dioxide:
Li2CO3 → Li2O + CO2
Reaction with lime produces lithium hydroxide:
Li2CO3+ Ca(OH)2→2LiOH + CaCO3
The carbonate reacts with molten aluminum fluoride converting to lithium fluoride:
3Li2CO3+ 2AlF3 → 6LiF + 3CO2+ Al2O3
It combines with carbon dioxide in aqueous slurry forming soluble bicarbonate, which decomposes to carbonate upon heating:
Li2CO3+ CO2+ H2O →2LiHCO3
The bicarbonate can not be separated in solid form. It exists only in solution when carbonate dissolves in water saturated with CO2under pressure.
Description
Lithium carbonate is a white hygroscopicpowder. Molecular weight = 73.89; Boiling point = 1310℃(decomposes below BP); Freezing/Meltingpoint = 618-735℃. Hazard Identification (based onNFPA-704 M Rating System): Health 1, Flammability 0,Reactivity 1. Slightly soluble in water.
Chemical Properties
Lithium carbonate is a white hygroscopic powder.
Physical properties
White monoclinic crystals; refractive index 1.428; density 2.11 g/cm3; melts at 723°C; decomposes at 1,310°C; low solubility in water (1.54 g/100g) at 0°C; 1.32 g//100g at 20°C), solubility decrease with temperature (0.72g/100g at 100°C); insoluble in acetone and ethanol.
Uses
Lithium carbonate is used as a compound for producing metallic lithium. Lithium carbonate is the result of treating the mineral spodumene with sulfuric acid and then adding calcium carbonate. It is used as an antidepressant.
Uses
In the production of glazes on ceramic and electrical porcelain.
Uses
The most common lithium drug is lithium carbonate, which possesses antimania action. It is presumed that lithium alters the transport of sodium ions in neurons, thus influencing the intercellular contents of catecholamines, normalizing the mental state and not causing general lethargy. It is used for mania conditions of various origins, preventative measures, and for treating affective psychoses.
Indications
Lithium inhibits thyroidal incorporation of I- into Tg, as well as the secretion of thyroid hormones, but it does not inhibit the activity of the Na+-I- symporter or the accumulation of I- within the thyroid. Lithium offers no particular advantage over drugs of the thionamide class but may be employed for temporary control of thyrotoxicosis in patients who are allergic to both thionamides and iodide.
Preparation
Lithium carbonate is prepared by the precipitation of lithium ion by carbonate ion from an aqueous solution. Still another process, which is carried out on a smaller scale, is the reaction of a solution of lithium hydroxide with carbon dioxide gas. Lithium carbonate precipitates and is recovered from the supernatant solution.
Definition
lithium carbonate: A white solid,Li2CO3; r.d. 2.11; m.p. 723°C; decomposesabove 1310°C. It is producedcommercially by treating the ore with sulphuric acid at 250°C andleaching the product to give a solutionof lithium sulphate. The carbonateis then obtained by precipitationwith sodium carbonate solution.Lithium carbonate is used in the preventionand treatment of manicdepressivedisorders. It is also usedindustrially in ceramic glazes.
brand name
Eskalith (GlaxoSmithKline); Lithane (Bayer); Lithobid (JDS); Lithonate (Solvay Pharmaceuticals).
General Description
Lithiumcarbonate (Eskalith, Lithane) and lithium citrate(Cibalith-S) are the salts commercially available in theUnited States.
Reactivity Profile
A base. Decomposed by acids with the evolution of carbon dioxide. Fluorine burns fiercely on contact with Lithium carbonate.
Flammability and Explosibility
Non flammable
Clinical Use
Treatment and prophylaxis of mania, manic
depressive illness, and recurrent depression
Aggressive or self-mutilating behaviour
Side effects
Drowsiness, dizziness, tiredness, increased thirst, increased frequency of urination, weight gain, and mildly shaking hands (fine tremor) may occur. These should go away as your body adjusts to the medication. This medication may increase serotonin and rarely cause a very serious condition called serotonin syndrome/toxicity. The risk increases if you are also taking other drugs that increase serotonin, so tell your doctor or pharmacist of all the drugs you take (see Drug Interactions section). Get medical help right away if you develop some of the following symptoms: fast heartbeat, hallucinations, loss of coordination, severe dizziness, severe nausea/vomiting/diarrhea, twitching muscles, unexplained fever, unusual agitation/restlessness.
Safety Profile
Human carcinogenic data. Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion and subcutaneous routes. Human systemic effects by ingestion: toxic psychosis, tremors, changes in fluid intake, muscle weakness, increased urine volume, nausea or vomiting, allergic dermatitis. Human reproductive effects by ingestion: effects on newborn, including Apgar score changes and other neonatal measures or effects. Human teratogenic effects by ingestion: developmental abnormalities of the cardiovascular system, central nervous system, musculoskeletal and gastrointestinal systems. An experimental teratogen. Experimental reproductive effects. Experimental carcinogen producing leukemia and thyroid tumors. Human mutation data reported. Used in the treatment of manic-depressive psychoses. Incompatible with fluorine. See also LITHIUM COMPOUNDS.
Synthesis
Lithium carbonate is synthesized by reacting lithium salts with soda or potash, followed by purification of the salt, which is not readily soluble [75].
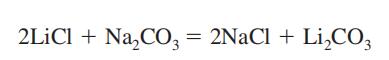
Potential Exposure
Lithium carbonate is used in treatment of manic-depressive psychoses; to make ceramics and porcelain glaze; varnishes, dyes, pharmaceuticals, coating of arc-welding electrodes; battery alloys; nucleonics, luminescent paints; glass ceramics; lubricating greases; in aluminum production
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
storage
Store at RT
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Purification Methods
Crystallise it from water. Its solubility decreases as the temperature is raised. The solubility in H2O is 1.3% at ~10o, and 0.7% at ~100o. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 987 1963, Caley & Elving Inorg Synth I 1 1939.]
Incompatibilities
The aqueous solution is a strong base. Reacts violently with acids, powdered calcium and fluorine.Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong acids, powdered calcium, fluorine, moisture. Corrodes aluminum, copper, zinc.
Lithium carbonate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 | catherine@yjchem.com.cn | China | 985 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57505 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 52925 | 58 |
| ChemFine International Co.,Ltd. | +86-510-82753588 +86-13806194144 | info@chemfineinternational.com | China | 300 | 58 |
| Hengshui Haoye Co.,Ltd. | +86-2102300 +86-18632882519 | hy@haoyecom.cn | China | 299 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 | admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 12272 | 58 |
| Runte International Trade Limited | 19565631292 19565631292 | lucky@sdruntechem.com | China | 270 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
Related articles
- Lithium Carbonate: A Critical Compound in Modern Chemistry
- Lithium carbonate, is an inorganic compound of considerable importance in various industries, particularly in the fields of m....
- Oct 25,2024
- Different applications of lithium carbonate
- Lithium carbonate(Li2CO3) is an inorganic compound.
- Jun 15,2022
View Lastest Price from Lithium carbonate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-27 | Lithium carbonate
554-13-2
|
US $0.00 / Kg/Drum | 1KG | 99%min, medical grade/industrial grade/battery grade | 1000kg | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-27 | Lithium carbonate
554-13-2
|
US $999.00-666.00 / ton | 1ton | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-11-27 | Lithium carbonate
554-13-2
|
US $6.00 / kg | 1kg | 99% | 2000KG/Month | HebeiShuoshengImportandExportco.,Ltd |
-

- Lithium carbonate
554-13-2
- US $0.00 / Kg/Drum
- 99%min, medical grade/industrial grade/battery grade
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Lithium carbonate
554-13-2
- US $999.00-666.00 / ton
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Lithium carbonate
554-13-2
- US $6.00 / kg
- 99%
- HebeiShuoshengImportandExportco.,Ltd