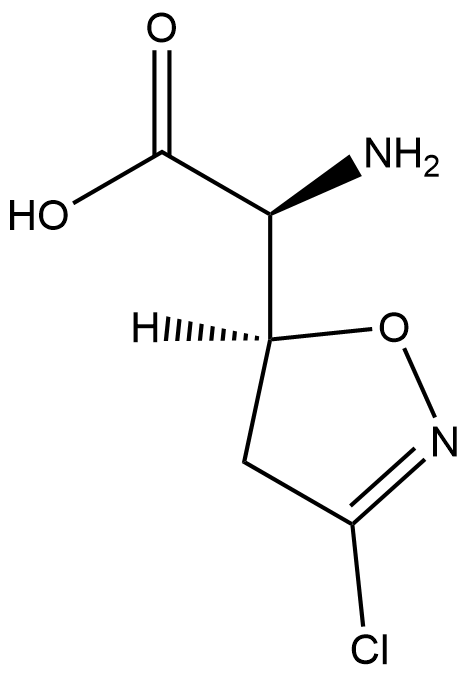
ACIVICIN
- CAS No.
- 42228-92-2
- Chemical Name:
- ACIVICIN
- Synonyms
- AT-125;U-42126;(αS,5S)-;ACIVICIN;acivicine;nsc-163501;antibioticat125;AcivicinAcivicin;Antibiotic U-42126;DIHYDROXY-5-ISOXAZOLEACETICACID
- CBNumber:
- CB6242253
- Molecular Formula:
- C5H7ClN2O3
- Molecular Weight:
- 178.57
- MDL Number:
- MFCD00866432
- MOL File:
- 42228-92-2.mol
| Melting point | >200°C (dec.) |
|---|---|
| Boiling point | 341.6±48.0 °C(Predicted) |
| Density | 1.85±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble10mg/mL (warmed) |
| pka | 2.02±0.10(Predicted) |
| form | White solid. |
| color | white to beige |
| Water Solubility | Soluble in water at 10mg/ml with warming |
| Stability | Hygroscopic |
| FDA UNII | O0X60K76I6 |
| NCI Drug Dictionary | acivicin |
ACIVICIN price More Price(19)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Alfa Aesar | J63105 | Acivicin, 98+% | 42228-92-2 | 10mg | $525 | 2024-03-01 | Buy |
| Alfa Aesar | J63105 | Acivicin, 98+% | 42228-92-2 | 25mg | $1295.65 | 2024-03-01 | Buy |
| Cayman Chemical | 14003 | Acivicin ≥98% | 42228-92-2 | 1mg | $50 | 2024-03-01 | Buy |
| Cayman Chemical | 14003 | Acivicin ≥98% | 42228-92-2 | 5mg | $200 | 2024-03-01 | Buy |
| Cayman Chemical | 14003 | Acivicin ≥98% | 42228-92-2 | 10mg | $276 | 2024-03-01 | Buy |
ACIVICIN Chemical Properties,Uses,Production
Chemical Properties
Off-White Solid
Originator
Acivicin ,ZYF Pharm Chemical
Uses
Inhibits glutamine amidotransferases in purine and pyrimidine synthetic pathways. Tumor growth inhibitor
Uses
Azaserine and Acivicin are classical and irreversible inhibitors of γ-Glutamyltranspeptidase
Definition
ChEBI: An L-alpha-amino acid that is L-alanine in which the methyl group is replaced by a (5S)-3-chloro-4,5-dihydro-1,2-oxazol-5-yl group. A glutamine analogue antimetabolite, it interferes with lutamate metabolism and several glutamate-dependent synthetic enzymes. It is obtained as a fermentation product of Streptomyces sviceus bacteria.
Manufacturing Process
Starting from commercial, cis-2-buten-1,4-diol, the monotrichloroacetimidate was obtained as a colorless liquid (60%, b.p. 88°-102°C/0.2 mm Hg) by treatment with trichloroacetonitrile (1 equivalent) in tetrahydrofuran at -23°C in the presence of catalytic amount of sodium. Monotrichloroacetimidate upon refluxing in tert-butyl benzene for about 1 hour underwent, smoothly,rearrangement to afford the vinylglycine synton (84%, MP: 30°C). The treatment of the last compound with bromonitrile oxide (3 equiv.) generated in situ from dibromoformaldoxime in ethyl acetate containing excess of KHCO3 and trace amounts of water afforded 3:2 mixture of cycloadducts threo- and erythro-N-[1-(3-bromo-4,5-dihydroisoxazol-5-yl)-2-hydroxyethyl]-2,2,2- trichloroacetamide. The undesired threo- isomer (MP: 164°-165°C) was quantitatively removed from the mixture by fractional crystallization from chloroform. The erythro-isomer (oil) was refluxed with methanolic-HCl for 1 hour to give the chloro-alchohol (50%, syrup), which upon Jones oxidation (with H2Cr2O7/acetone) followed by deprotection of trichloroacetyl group (Ba(OH)2/H2O, H3+O) afforded racemic acivicin (66 %). The synthetic, racemic antibiotic was spectrally (UV, 1H NMR) indistinguishable from
Therapeutic Function
Antineoplastic
Enzyme inhibitor
This cytotoxic isoxazole and copper chelator (FW = 178.57 g/mol; CAS 42228-92-2; Source: Streptomyces sviceus), also known as L-(aS,5S)-aamino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid, AT-125, and NSC165301, strongly inhibitsγ-glutamyl transpeptidase. Its clinical application in cancer treatment failed as a consequence of unacceptable toxicity, and the cause(s) of the desired and undesired biological effects have never been elucidated and only limited information about acivicin-specific targets is available. Target deconvolution by quantitative mass spectrometry (MS) has now revealed acivicin’s preference for the specific aldehyde dehydrogenase known as ALDH4A1 by binding to the catalytic site. Moreover, siRNA-mediated downregulation of ALDH4A1 results in a severe inhibition of cell growth, a finding that may explain acivicin’s cytotoxicity. Targets: Acivicin is thought to be a glutamine analogue, an assumption that is amply supported by its ability to inhibit the following enzymes that possess essential amidohydrolase activities that generate nascent ammonia: asparagine synthetase; carbamoyl-phosphate synthetase; anthranilate synthase; glutamate synthase; CTP symthetase; amidophospho-ribosyltransferase; glutamin(asparagin)ase, or glutaminase-asparaginase; GMP synthase, glutamine-dependent; phosphoribosyl-formylglycinamidine synthetase (formylglycinamidine ribonucleotide synthetase; thiol oxidase; γ-glutamyl hydrolase; imidazole-glycerol phosphate synthetase.
ACIVICIN Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39916 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | laboratory@coreychem.com | China | 30253 | 58 |
| BOC Sciences | 16314854226; +16314854226 | inquiry@bocsci.com | United States | 19743 | 58 |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | sales@nextpeptide.com | China | 19915 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131980 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 94717 | 76 |
| Chemsky(shanghai)International Co.,Ltd. | 021-50135380 | shchemsky@sina.com | China | 32344 | 50 |
| Shanghai Aladdin Bio-Chem Technology Co.,LTD | 400-400-6206333 18521732826 | market@aladdin-e.com | China | 25014 | 65 |