Mizoribine
- CAS No.
- 50924-49-7
- Chemical Name:
- Mizoribine
- Synonyms
- HE 69;bredinin;Mizorbine;bredinine;MIZORIBINE;Mizoribina;b-Bredinin;NSC 289637;Brn 4151713;Mizoribine RS
- CBNumber:
- CB6426440
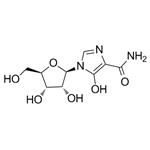
- Molecular Formula:
- C9H13N3O6
- Molecular Weight:
- 259.22
- MDL Number:
- MFCD00057221
- MOL File:
- 50924-49-7.mol
- MSDS File:
- SDS
| Melting point | >2000C |
|---|---|
| alpha | D27 -35° (c = 0.8 in H2O) |
| Boiling point | 402.47°C (rough estimate) |
| Density | 1.4155 (rough estimate) |
| refractive index | 1.5100 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Water (Slightly) |
| form | Solid |
| pka | 6.75(at 25℃) |
| color | White to Off-White |
| λmax | 280nm(lit.) |
| Merck | 14,6222 |
| InChIKey | HZQDCMWJEBCWBR-UUOKFMHZSA-N |
| CAS DataBase Reference | 50924-49-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 4JR41A10VP |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H319-H335-H360 | |||||||||
| Precautionary statements | P201-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 46-60-61-36/37/38 | |||||||||
| Safety Statements | 53-22-26-36/37/39-45 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | NI3980000 | |||||||||
| HS Code | 29349990 | |||||||||
| Toxicity | LD50 in mice (g/kg): >1.5 i.v., >2.4 i.p. (Mizuno, 1975) | |||||||||
| NFPA 704 |
|
Mizoribine price More Price(48)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | M3047 | Mizoribine ≥98% (TLC) | 50924-49-7 | 25mg | $137.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | M3047 | Mizoribine ≥98% (TLC) | 50924-49-7 | 10mg | $158 | 2024-03-01 | Buy |
| TCI Chemical | M2399 | Mizoribine >98.0%(HPLC) | 50924-49-7 | 50mg | $180 | 2024-03-01 | Buy |
| TCI Chemical | M2399 | Mizoribine >98.0%(HPLC) | 50924-49-7 | 250mg | $623 | 2024-03-01 | Buy |
| Cayman Chemical | 23128 | Mizoribine ≥95% | 50924-49-7 | 10mg | $40 | 2024-03-01 | Buy |
Mizoribine Chemical Properties,Uses,Production
Outline
Mizoribine is a kind of imidazole nucleoside isolated from the culture medium of ascomycetes M-2166 separated from the soil by Japanese scholars Asahi Kasei, being the metabolite which inhibiting the purine synthesis pathway of nucleic acid, mainly being used for inhibiting the rejection reaction before or after renal transplantation and also used for liver transplantation and autoimmune diseases.
Mizoribine, as a kind of immunosuppressant, its immunosuppressive effects may be related to its inhibition of purine nucleotide synthesis and increased transcriptional activity of the glucocorticoid receptor. It has been applied at Japanese clinical renal transplantation since December 1991. Many Japanese clinical transplant centers have applied mizoribine as the routine immunization inhibitory drug after kidney transplantation. In recent year, China has also applied kidney transplant anti-rejection drugs for clinical purpose. Mizoribine, compared with similar drugs azathioprine, has a low liver toxicity and bone marrow suppression effect with its main adverse reaction being the gastrointestinal reactions, blood disorders and allergic symptoms, and occasionally bone marrow suppression and acute renal failure.
Pharmacological effects
This product is imidazole nucleoside anti-metabolite drug. Mizoribine, as a kind of pro-drugs, needs to undergo intracellular phosphorylation before taking effect. Its mechanism of immune suppression is mainly through inhibition of inosine monophosphate dehydrogenase (IMPDH) and guanosine monophosphate synthetase (GMP), and reducing the synthesis of guanylate as well as the synthesis of RNA, DNA, thereby inhibiting the proliferation of lymphocyte and the production of antibody, prolonging the survival time after organ transplantation, preventing the cell to be transferred from G1 to S phase. Because lymphocyte is not capable of synthesizing purine via salvage pathway, this product is able to selectively inhibit its activation. In addition, the product can interfere with the expression of cytokine receptors and antagonize the activation of lymphocyte mediated by cytokine. This product has a milder myelosuppression effect compared with azathioprine. The bioavailability of oral administration is relative low with large differences in individuals. After oral administration, the average time of plasma concentration for reaching peak is about 3 to 4 hours. This product is widely distributed in each body tissue with a higher concentration than that in the blood and with the highest concentration in the kidney and stomach. It is not easy to penetrate through the blood-brain barrier, but can penetrate through the placental barrier with a small amount shifting to the breast milk. It is mainly excreted through renal in its prototype with the elimination half-life being 2 to 5 hours.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Pharmacodynamics
This product is able to inhibit the gastric transplant rejection with pharmacological effect of specifically inhibiting the proliferation of lymphocyte, and blocking the embryo-like conversion reaction induced by various kinds of mitogenic factors induced embryo-like conversion reaction, being able to extend the life of transplanted organs. It can inhibit the biosynthesis of nucleic acid through antagonizing the purine synthesis process from the pathway of inosine monophosphate to guanosine acid pathway without penetrating the nucleic acid polymer.
Side effects
Mizoribine has bone marrow suppression effects such as causing reduction of white blood cells, platelets, and red blood cells. Occasionally there may also be liver damage, elevated blood urea nitrogen, gastrointestinal bleeding, allergies, fever, hair loss, lung infections, glossitis, and stomatitis. There are also loss of appetite, nausea, vomiting, diarrhea, and abdominal swelling feeling.
Precautions
1. Patients allergic to this drug as well as with their white blood cell count being lower than 3 × 109/L, as well as pregnant women, lactating women should be disabled. Patients of bone marrow suppression, accompanied postoperative bacterial or viral infections, bleeding tendency, liver and kidney dysfunction should be used with caution.
2. The safety of children, women of childbearing age for using this drug has not been established.
3. We should pay close attention to the treatment of bleeding and infections during the treatment period. Moreover, we should perform regular investigation on the thermograms and liver and kidney function and discontinue or adjust the dose according to the medication condition.
Category
toxic substances
Toxicity grading
poisoning
Acute toxicity
oral-rat LD50: 3100 mg/kg; intraperitoneal-Mouse LD50: 5000 mg/kg
Flammability and hazard properties
Thermal decomposition can discharge toxic nitrogen oxides gases
Storage characteristics
Treasury: ventilation, cool, dry
Extinguishing media
Water, foam, sand, carbon dioxide, dry powder
Description
Mizoribine is an orally active immunosuppressant isolated from Eupenicillium brefeldianum. It is reported to inhibit both cell-mediated and antibody mediated immune responses. Mizoribine is approved for use in the prevention of rejection of kidney transplants.
Description
Mizoribine is an imidazole nucleoside with immunosuppressive properties. It inhibits T cell proliferation in response to various mitogenic stimuli by 10-100% when used at concentrations ranging from 1 to 50 μg/mL. Mizoribine inhibits proliferation of stimulated T cells (IC50 = 5 μg/ml), which can be reversed by guanosine. It also inhibits guanine nucleotide formation in T cells, reducing GTP pools by 40-60% when used at a concentration of 5 μg/ml. Mizoribine inhibits replication of hepatitis C virus (HCV) RNA in vitro (IC50 = 100 μM). It suppresses glomerulosclerosis, urinary albumin excretion, interstitial fibrotic lesions, and macrophage infiltration into glomeruli and the interstitium in a rat model of type 2 diabetes when used at doses of 5 or 10 mg/kg. Mizoribine also reduces MCP-1, osteopontin (OPN), and TGF-β1 mRNA expression in the kidney in the same model. Formulations containing mizoribine have been used for the prevention of rejection after renal transplantation as well as in the treatment of lupus nephritis, rheumatoid arthritis, and primary nephritic syndrome.
Chemical Properties
Crystalline Solid
Originator
Toyo Jozo (Japan)
Uses
Mizoribine has been used in topical treatment to evaluate its effect on ocular surface damage of dry eye in B6 mice subjected to desiccating stress (DS). It has also been used as an inosine monophosphate dehydrogenase (IMPDH) inhibitor to test its in vivo efficacy.
Uses
Nucleoside antibiotic with cytotoxic and immunosuppressive activity
Definition
ChEBI: Mizoribine is a member of imidazoles. It has a role as an anticoronaviral agent.
brand name
BREDININ
Biochem/physiol Actions
Mizoribine is an imidazole nucleoside possessing strong immunosuppressive properties. It selectively blocks T-cell proliferation response to mitogenic and allo-antigenic stimulation. Mizoribine blocks the movement of T cells from G to S phase. In addition, it significantly decreases the number of B cells at the S, G, and M phases. Mizoribine inhibits de novo synthesis of nucleotides by inhibition of inosine monophosphate dehydrogenase. The resulting nucleotide depletion inhibits DNA synthesis.(3)
Mizoribine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Apeloa production Co.,Limited | +8619933239880 | admin@apcl.com.cn | China | 861 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29730 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21630 | 55 |
| Shanghai finete Pharmaceutical Co., Ltd. | +86-18221039705 | CHINA | 139 | 55 | |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29862 | 58 |
| Shanghai Arbor Chemical Co., Ltd. | 021-60451682 | act@arborchemical.com | CHINA | 904 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8615060885618 | sales@amoychem.com | China | 6383 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19552 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39894 | 58 |
| Shenzhen Excellent Biotech Co., Ltd. | 13480692018 | ramyan@ex-biotech.com | CHINA | 954 | 58 |
View Lastest Price from Mizoribine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-11 | Mizoribine
50924-49-7
|
US $0.00 / g | 1g | 98%min | 50kg | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2025-03-21 | Mizoribine
50924-49-7
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mujin Biotechnology Co.,Ltd | |
 |
2025-02-13 | Mizoribine
50924-49-7
|
US $1.00 / g | 1g | 0.98 | 1000 | Apeloa production Co.,Limited |
-

- Mizoribine
50924-49-7
- US $0.00 / g
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Mizoribine
50924-49-7
- US $0.00 / KG
- 99%
- Hebei Mujin Biotechnology Co.,Ltd
-

- Mizoribine
50924-49-7
- US $1.00 / g
- 0.98
- Apeloa production Co.,Limited





