ALDICARB-SULFONE
- CAS No.
- 1646-88-4
- Chemical Name:
- ALDICARB-SULFONE
- Synonyms
- ALDOXYCARB;UCES;ent4.9;Standak;STANDOK;UC21865;Sulfocarb;STANDAK(R);aldoxycarbe;YangdiMiewei
- CBNumber:
- CB7710151
- Molecular Formula:
- C7H14N2O4S
- Molecular Weight:
- 222.26
- MDL Number:
- MFCD00041804
- MOL File:
- 1646-88-4.mol
- MSDS File:
- SDS
| Melting point | 132-135 °C |
|---|---|
| Density | 1.3816 (rough estimate) |
| vapor pressure | 1.2×10-2 Pa (25 °C) |
| refractive index | 1.5690 (estimate) |
| storage temp. | 0-6°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| Water Solubility | 10 g l-1 (25 °C) |
| pka | 13.44±0.46(Predicted) |
| form | Solid |
| color | White to Off-White |
| BRN | 2215242 |
| EWG's Food Scores | 1 |
| FDA UNII | IL70ANS043 |
| EPA Substance Registry System | Aldicarb sulfone (1646-88-4) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H400 | |||||||||
| Precautionary statements | P260-P262-P273-P280-P302+P352+P310-P304+P340+P310 | |||||||||
| Hazard Codes | T+ | |||||||||
| Risk Statements | 24-26/28 | |||||||||
| Safety Statements | 28-36/37-45 | |||||||||
| RIDADR | 2757 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | UE2080000 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | I | |||||||||
| Toxicity | duck,LD50,oral,33500ug/kg (33.5mg/kg),Pesticide Manual. Vol. 9, Pg. 18, 1991. | |||||||||
| NFPA 704 |
|
ALDICARB-SULFONE price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 33387 | Aldicarb-sulfone PESTANAL | 1646-88-4 | 100mg | $171 | 2024-03-01 | Buy |
| Cayman Chemical | 24210 | Aldicarb sulfone ≥98% | 1646-88-4 | 50mg | $72 | 2024-03-01 | Buy |
| Cayman Chemical | 24210 | Aldicarb sulfone ≥98% | 1646-88-4 | 25mg | $40 | 2023-06-20 | Buy |
| Cayman Chemical | 24210 | Aldicarb sulfone | 1646-88-4 | 100mg | $130 | 2024-03-01 | Buy |
| American Custom Chemicals Corporation | PST0000340 | ALDICARB-SULFONE 98.00% | 1646-88-4 | 100MG | $750.75 | 2021-12-16 | Buy |
ALDICARB-SULFONE Chemical Properties,Uses,Production
Description
Aldoxycarb, is also called 2-mesyl-2-methylpropionaldehyde O-methylcarbamoyloxime (IUPAC), the sulfone analog of aldicarb, is also a systemic insecticide effective for control of insects (mainly sucking insect pests such as aphids, leafhoppers, etc.).
Chemical Properties
Aldoxycarb is a carbamate pesticide used mainly as an insecticide and acaricide. It is a white crystalline powder with a slightly sulfurous odor. Aldoxycarb is extremely toxic to wildlife. Aldoxycarb (Aldicarb sulfone) is a breakdown product of aldicarb. Aldoxycarb is a degradate/metabolite of aldicarb produced by the oxidation of the thio-moiety.
Uses
Aldoxycarb is a soil applied systemic oxime carbamate insecticide effective for the control of insects (mainly sucking pests such as aphids, leafhoppers, etc.), mites and nematodes on many field (cotton, maize, etc.) and vegetable crops.
Uses
Aldicarb Sulfone is an analog of Aldicarb (A514650) detected in agricultural products as pesticide residue.
Definition
ChEBI: Aldoxycarb is a sulfone.
General Description
White crystalline solid. Insecticide.
Reactivity Profile
ALDICARB-SULFONE is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates.
Health Hazard
Exposures to high levels of aldoxycarb cause adverse health effects and damages the nervous system of humans and laboratory animals. Chemicals that cause adverse health effects in humans and laboratory animals after high levels of exposure may pose a risk of adverse health effects in humans exposed to lower levels over long periods of time. Ingestion and other exposures to the chemical cause various health disorders. The type and severity of symptoms varies depending on the amount of chemical involved and the nature of the exposure. The symptoms include, but are not limited to, eye irritation, skin irritation, dizziness, sweating, malaise, CNS depression, abdominal pain, diarrhea, nausea, vomiting, salivation, headache, ataxia, muscle twitching, muscle weakness, runny nose, tearing eyes, pinpoint pupils or dark vision, wheezing, increased or decreased heart rate, seizures, convulsions, and death. It has not caused any delayed neurotoxicity in animals. The onset of these symptoms is rapid and the severity of symptoms depends on the dose. The immediate cause of death is usually respiratory failure.
Health Hazard
Highly toxic by all routes of exposure;cholinesterase inhibitor; ingestion of 1–3 gmay cause death to adult humans.
LD50 oral (mouse): 20 mg/kg
LD50 inhalation (rat): 140 mg/m3/4 hr
LD50 skin (rabbit): 200 mg/kg.
Agricultural Uses
Insecticide, Acaricide, Nematicide: Used to control honey locust, gall midge and other insects and mites on cotton, potatoes, sugar beets and ornamentals. Applied to the soil by soluble mixture or spikes. Not listed for use in EU countries.
Trade name
ALDICARB SULFONE®; TEMIK SULFONE®; SULFOCARB®; STANDAK®; UC-21865®
Metabolic pathway
Aldoxycarb is the sulfone analogue of aldicarb. It has now been superseded but, because of its close relationship with aldicarb, it is included here. The degradation and metabolic pathway of these two structurally related compounds are consistent with each other. Information on the metabolism of aldoxycarb discussed in this summary is based on results obtained from studies where aldoxycarb was the test substance. Additional information can be obtained from the aldicarb summary. The metabolism of aldoxycarb in soils, plants and animals follow common metabolic pathways which include the hydrolysis and elimination of the oxime carbamate to yield the corresponding oximes and nitriles. These primary metabolites are further degraded via hydrolysis to the corresponding amides and acids. Other reactions also include the hydrolysis/ oxidation of the oxime to the corresponding alcohol, aldehyde and acid. Further degradation to methane sulfonic acid was observed in plants and animals. The metabolic pathways of aldoxycarb are presented in Scheme 1.
Degradation
Aldoxycarb (1) is susceptible to alkaline hydrolysis. It was stable under acidic (pH 5) conditions, slowly degraded (DT50 82 days) in neutral conditions (pH 7) and rapidly degraded (DT50 0.8 days) under basic (pH 9) conditions (Andrawes, 1976a). Aldoxycarb oxime (2), aldoxycarb nitrile (3) and methanesulfonic acid (4) were the major hydrolysis products. Aldoxycarb degraded slowly in water under photolytic conditions (xenon lamp) with a half-life of approximately 38 days (Andrawes, 1976b) to yield aldoxycarb nitrile (3) as the major degradation product.
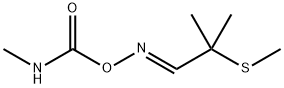
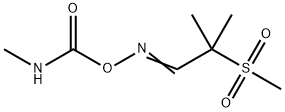
ALDICARB-SULFONE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63687 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29808 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32159 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6312 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | sales@afinechem.com | China | 15350 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8740 | 58 |
| TargetMol Chemicals Inc. | support@targetmol.com | United States | 38630 | 58 | |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 | sales@senfeida.com | China | 26362 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +86-18621343501; +undefined18621343501 | product@acmec-e.com | China | 33338 | 58 |
View Lastest Price from ALDICARB-SULFONE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-19 | Aldicarb sulfone
1646-88-4
|
US $50.00 / mL | 99.05% | 10g | TargetMol Chemicals Inc. | ||
 |
2020-02-01 | Aldicarb (sulfone)
1646-88-4
|
US $1.00 / KG | 1KG | Min98% HPLC | g/kg/ton | Career Henan Chemical Co |
-

- Aldicarb sulfone
1646-88-4
- US $50.00 / mL
- 99.05%
- TargetMol Chemicals Inc.
-

- Aldicarb (sulfone)
1646-88-4
- US $1.00 / KG
- Min98% HPLC
- Career Henan Chemical Co





