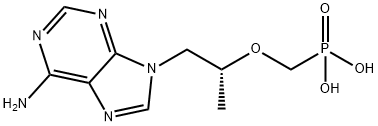
Tenofovir
- CAS No.
- 147127-20-6
- Chemical Name:
- Tenofovir
- Synonyms
- (R)-9-(2-Phosphonomethoxypropyl)Adenine;Viread;GS-1278;Apropovir;(R)-9-(2-PHOSPHONYLMETHOXYPROPYL)-ADENINE;9-Pmpa;GS 1275;(R)-PMPA;Tenofovi;enofovir
- CBNumber:
- CB8218508
- Molecular Formula:
- C9H14N5O4P
- Molecular Weight:
- 287.21
- MDL Number:
- MFCD07357269
- MOL File:
- 147127-20-6.mol
- MSDS File:
- SDS
| Melting point | 276-280°C |
|---|---|
| alpha | D +21° (c = 1 in 0.1M HCl) |
| Boiling point | 616.1±65.0 °C(Predicted) |
| Density | 1.79±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Aqueous Acid (Sparingly), DMSO (Slightly, Heated), Water (Slightly, Heated) |
| pka | 2.36±0.10(Predicted) |
| form | powder |
| color | white to beige |
| optical activity | [α]/D -20 to -26°, c = 0.5 in 1 M HCl |
| Water Solubility | 13.4 mg/mL (25 ºC) |
| Merck | 14,9146 |
| InChI | InChI=1S/C9H14N5O4P/c1-6(18-5-19(15,16)17)2-14-4-13-7-8(10)11-3-12-9(7)14/h3-4,6H,2,5H2,1H3,(H2,10,11,12)(H2,15,16,17)/t6-/m1/s1 |
| InChIKey | SGOIRFVFHAKUTI-ZCFIWIBFSA-N |
| SMILES | P(CO[C@H](C)CN1C2C(N=C1)=C(N)N=CN=2)(=O)(O)O |
| CAS DataBase Reference | 147127-20-6(CAS DataBase Reference) |
| FDA UNII | W4HFE001U5 |
| NCI Drug Dictionary | tenofovir |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H332-H335 | |||||||||
| Precautionary statements | P261-P280-P305+P351+P338 | |||||||||
| RTECS | SZ6563600 | |||||||||
| HS Code | 29339900 | |||||||||
| NFPA 704 |
|
Tenofovir price More Price(57)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML1795 | Tenofovir ≥98% (HPLC) | 147127-20-6 | 5MG | $135 | 2023-06-20 | Buy |
| Sigma-Aldrich | SML1795 | Tenofovir ≥98% (HPLC) | 147127-20-6 | 25MG | $542 | 2023-06-20 | Buy |
| TCI Chemical | T3006 | Tenofovir Hydrate >98.0%(HPLC)(T) | 147127-20-6 | 1g | $54 | 2024-03-01 | Buy |
| TCI Chemical | T3006 | Tenofovir Hydrate >98.0%(HPLC)(T) | 147127-20-6 | 5g | $185 | 2024-03-01 | Buy |
| Cayman Chemical | 13874 | Tenofovir ≥98% | 147127-20-6 | 5mg | $81 | 2024-03-01 | Buy |
Tenofovir Chemical Properties,Uses,Production
Uses
Tenofovir is an acyclic phosphonate nucleotide analogue and reverse transcriptase inhibitor. It is used as an anti-HIV agent. Antiviral.
Indications and Usage
Tenofovir disoproxil (Viread) is the first nucleotide analogue approved by the American FDA to treat HIV-1 infections. Tenofovir disoproxil is a drug used in the AIDS cocktail treatment method, and research shows that it has the ability to increase monkeys’ immunity to immunodeficiency viruses (similar to the human AIDS virus). Tenofovir disoproxil is used in combination with other reverse transcriptase inhibitors to treat HIV-1 infections and hepatitis B.
Mechanisms of Action
Tenofovir disoproxil is an acyclic nucleoside antivirus drug and has an inhibiting effect on HBV multi-enzyme complexes and HIV reverse transcriptase. Its active content tenofovir phosphonate directly competitively binds to natural deoxyribose substrate to inhibit the virus multi-enzyme complex and inserts itself into the DNA to end the nucleotide chain. Tenofovir disoproxil is barely absorbed by the gastrointestinal duct, so it undergoes esterification and ionization to become tenofovir ester fumarate. Tenofovir is soluble in water and can be quickly absorbed and decomposed into the active substance tenofovir, which then transforms into the active metabolite tenofovir phosphonate. As this drug is not metabolized by the CYP450 enzyme system, it has a very low chance of drug interactions caused by this enzyme.
Pharmacokinetics
Tenofovir disoproxil reaches peak blood concentration 1-2 hours after intake. Tenofovir disoproxil’s bioavailability increases by about 40% when taken with food. The intracellular half-life of tenofovir phosphonate is about 10 hours, so doses can be taken once daily. This drug is mainly filtered through renal glomeruli and excreted through the renal tubule transport system, with 70-80% excreted in its original form through urine.
Adverse Effects
- Weakness and exhaustion.
- Mild to moderate gastrointestinal reactions, including diarrhea, stomach pain, nausea, vomiting, bloating, lactic acid poisoning, hepatomegaly and fatty liver, and pancreatitis. These adverse reactions also commonly appear individually or combined when taking nucleoside analogues.
- Metabolic system hypophosphatemia (1% occurrence rate).
- Fat accumulation and redistribution, including centripetal obesity, buffalo hump, thin limbs, breast growth, and Cushing syndrome.
- May cause lactic acid poisoning, hepatomegaly related to steatosis, etc.
- Effects on nervous system: dizziness and headache.
- Effects on respiratory system: difficulty breathing.
- Effects on skin: drug rash.
Description
Tenofovir is an analog of adenosine monophosphate that has antiviral activity. It is converted by cellular enzymes to tenofovir diphosphate, an obligate chain terminator that inhibits the activity of HIV reverse transcriptase and hepatitis B virus polymerase. Tenofovir diphosphate is a weak inhibitor of mammalian DNA polymerases α and β and mitochondrial DNA polymerase γ. For in vivo and cell culture use, tenofovir is supplied as a water soluble prodrug in the form of tenofovir disoproxil (fumarate) , which increases the intracellular diphosphorylated compound >1,000-
Chemical Properties
White Crystalline Solid
Uses
Tenofovir is a drug used for the treatment of chronic heptatitis B as well as prevention and treatment of HIV/AIDS. It is a kind of nucleotide analog, acting as the reverse-transcriptase inhibitor (NtRTI). It inhibits the activity of HIV reverse transcriptase through competing with the natural substrate deoxyadenosine 5’-triphosphate, causing the termination of DNA chain.
Uses
Tenofovir is an acyclic phosphonate nucleotide analogue and reverse transcriptase inhibitor. It is used as an anti-HIV agent. Antiviral.
Indications
Tenofovir disoproxil fumarate (Viread) is a prodrug of tenofovir, a phosphorylated adenosine nucleoside analogue, and is the only available agent of its class. It is converted by cellular enzymes to tenofovir diphosphate, which competes with deoxyadenosine triphosphate (dATP) for access to reverse transcriptase and causes chain termination following its incorporation. Tenofovir was approved as part of a combination therapy for HIV in adults who failed treatment with other regimens; it appears to be effective against HIV strains that are resistant to NRTIs.
Definition
ChEBI: A member of the class of phosphonic acids that is methylphosphonic acid in which one of the methyl hydrogens is replaced by a [(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy group. An inhibitor of HIV-1 reverse transcriptase, the bis(isopropyloxycarbonyloxy ethyl) ester (disoproxil ester) prodrug is used as the fumaric acid salt in combination therapy for the treatment of HIV infection.
Acquired resistance
HIV variants with the K65R mutation and the K70E mutation in the reverse transcriptase demonstrate reduced susceptibility to tenofovir.
Pharmaceutical Applications
An acyclic nucleoside phosphonate, formulated as the disoproxil fumarate salt for oral administration.
Pharmaceutical Applications
A nucleotide analog structurally similar to adefovir.
EC50 values for HBV, assessed in the HepG2 2.2.15 cell
line, ranged from 0.14 to 1.5 μm; the cytotoxic concentration
exceeded 100 μm. A decline in HBV DNA levels below
105 copies/mL at 48 weeks of therapy in 100% of patients
receiving tenofovir compared with 44% on adefovir therapy
has been reported. There are also case reports of patients with
primary resistance to adefovir responding to tenofovir.
It is generally well tolerated in patients with chronic HBV;
the most common side effects include nausea and gastrointestinal
upset, headache, dizziness, fatigue and rash.
Biological Activity
Selectively inhibits HIV reverse transcriptase (RNA-dependent DNA polymerase). Prevents cytotoxicity in SIV-infected C-8166 cells in vitro (IC 50 = 1.5 μ M). Antiviral agent.
Biochem/physiol Actions
Tenofovir has a low oral bioavailability. Hence, it is available as a prodrug called tenofovir disoproxil fumarate. Once ingested, tenofovir disoproxil fumarate is hydrolyzed to tenofovir and phosphorylated. This is then incorporated into the viral DNA which leads to chain termination. Tenofovir is also effective against hepatitis B virus.
Pharmacokinetics
Oral absorption: c. 25%
Cmax 300 mg once daily: 0.3 mg/L
Plasma half-life: 17 h
Volume of distribution: 1.3 ± 0.6 L/kg at 3.0 mg/kg
intravenous dose
Plasma protein binding: <0.7% (in vitro)
Absorption and distribution
Oral bioavailability is poor, but is enhanced by administration as the disoproxil prodrug. It may be taken with or without food. CSF penetration is likely to be minimal due to the anionic charge of the molecule at physiological pH. It accumulates in semen at higher concentrations than in plasma. It is not known if it is distributed into breast milk.
Metabolism and excretion
Tenofovir is not metabolized and is principally eliminated by the kidneys by a combination of glomerular filtration and active tubular secretion. In patients with renal dysfunction the dose should be adjusted accordingly.
Compounds such as cidofovir, aciclovir (acyclovir), valaciclovir, ganciclovir, valganciclovir and probenecid may compete for renal excretion. Tenofovir levels are increased when prescribed with some HIV protease inhibitors. The co-administration of tenofovir with didanosine leads to didanosine accumulation which is thought to occur through inhibition of purine nucleoside phosphorylase. This has been associated with impaired immune recovery and several cases of lactic acidosis and pancreatitis. If tenofovir is combined with didanosine the dose of didanosine should be reduced to 200 mg (<60 kg) or 250 mg (≥60 kg) per day and the patient monitored for symptoms of didanosine toxicity.
Clinical Use
Treatment of HIV infection in adults and children (in combination with other antiretroviral drugs)
Clinical Use
Chronic hepatitis B infection
Side effects
Tenofovir is taken once daily and is generally well tolerated, perhaps because it produces less mitochondrial toxicity than the NRTIs. Nausea, vomiting, flatulence, and diarrhea occur in 10% or fewer patients. Resistance to tenofovir has been documented, and cross-resistance to NRTIs may occur.
Side effects
In clinical trials of antiretroviral treatment-naive participants,
the most commonly reported adverse events were mild to
moderate gastrointestinal upset (nausea 8%, diarrhea 11%),
headache (14%) and depression (11%). Tenofovir has the
potential to result in nephrotoxicity, particularly through proximal
tubular damage, but the risk of clinically significant renal
dysfunction appears relatively low and seems to occur mainly
in subjects with other identifiable risks for renal impairment.
Minor elevations in serum creatinine and reductions in creatinine
clearance occur, but rarely require drug discontinuation.
A few (<0.1%) cases of osteomalacia and decreased bone
density have been reported.
storage
Store at -20°C
Tenofovir Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Yingrui Biopharma Co.,Ltd | 21-33585366 | export01@shyrchem.com | CHINA | 1319 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8812 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Sigma Audley | +86-15937194204 +86-18126314766 | nova@sh-teruiop.com | China | 467 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | sarah@tnjone.com | China | 1143 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29791 | 60 |
| Nanjing Gold Pharmaceutical Technology Co. Ltd. | 025-84209270 15906146951 | CHINA | 115 | 55 | |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1803 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21634 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | FandaChem@Gmail.com | China | 9214 | 55 |
Related articles
- What is Tenofovir ?
- Tenofovir is an acyclic nucleoside phosphonate. Tenofovir is manufactured as the prodrug tenofovir disoproxil fumarate (TDF) a....
- Apr 19,2022
View Lastest Price from Tenofovir manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Tenofovir
147127-20-6
|
US $0.00-0.00 / Kg/Drum | 1KG | 98%min HPLC | 500kgs | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-22 | Tenofovir
147127-20-6
|
US $0.00-0.00 / Kg/Bag | 0.1Kg/Bag | 99% min, GMP | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-11-01 | Tenofovir
147127-20-6
|
US $0.00 / KG | 1KG | 99 | 10Ton | Hebei Weibang Biotechnology Co., Ltd |