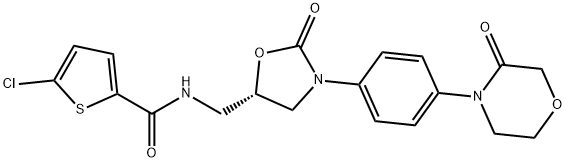
Rivaroxaban
- CAS No.
- 366789-02-8
- Chemical Name:
- Rivaroxaban
- Synonyms
- Xarelto;Rivaroxban;(S)-5-Chloro-N-((2-oxo-3-(4-(3-oxomorpholino)phenyl)-oxazolidin-5-yl)methyl)thiophene-2-carbox;(S)-5-chloro-N-((2-oxo-3-(4-(3-oxomorpholino)phenyl)oxazolidin-5-yl)methyl)thiophene-2-carboxamide;(S)-5-chloro-N-{[2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]oxazolidin-5-yl]methyl} thiophene-2-carboxamide;RIVAROXABAN STAGE-IV [5-CHLORO-N-({5S)-2-OXO-3-[4-(3-OXO-4-MORPHOLINYL)PHENYL]-1,3-OXAZOLIDIN-5-YL}-METHYL)-2(AS PER INV;CS-273;Rivaroxaba;Rivarobaxan;BAY 59-7939
- CBNumber:
- CB91176772
- Molecular Formula:
- C19H18ClN3O5S
- Molecular Weight:
- 435.88
- MDL Number:
- MFCD11974010
- MOL File:
- 366789-02-8.mol
| Melting point | 228-229°C |
|---|---|
| Boiling point | 732.6±60.0 °C(Predicted) |
| Density | 1.460±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | insoluble in H2O; insoluble in EtOH; ≥13.9 mg/mL in DMSO with gentle warming |
| form | solid |
| pka | 13.36±0.46(Predicted) |
| color | White to off-white |
| InChI | InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 |
| InChIKey | KGFYHTZWPPHNLQ-AWEZNQCLSA-N |
| SMILES | C1(C(NC[C@@H]2OC(=O)N(C3=CC=C(N4CCOCC4=O)C=C3)C2)=O)SC(Cl)=CC=1 |
| FDA UNII | 9NDF7JZ4M3 |
| NCI Drug Dictionary | rivaroxaban |
| ATC code | B01AF01 |
Rivaroxaban price More Price(51)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 16043 | Rivaroxaban ≥98% | 366789-02-8 | 1mg | $30 | 2024-03-01 | Buy |
| Cayman Chemical | 16043 | Rivaroxaban ≥98% | 366789-02-8 | 5mg | $67 | 2024-03-01 | Buy |
| Cayman Chemical | 16043 | Rivaroxaban ≥98% | 366789-02-8 | 10mg | $119 | 2024-03-01 | Buy |
| Cayman Chemical | 16043 | Rivaroxaban ≥98% | 366789-02-8 | 50mg | $444 | 2024-03-01 | Buy |
| TRC | R538000 | Rivaroxaban | 366789-02-8 | 1mg | $65 | 2021-12-16 | Buy |
Rivaroxaban Chemical Properties,Uses,Production
Description
Rivaroxaban(366789-02-8) is a pure (S)-enantiomer. It is an odorless, non-hygroscopic, white to yellowish powder. Rivaroxaban is only slightly soluble in organic solvents (e.g., acetone, polyethylene glycol 400) and is practically insoluble in water and aqueous media.
Rivaroxaban, a FXa inhibitor, is the active ingredient in XARELTO Tablets. XARELTO a kind of orally anticoagulant drug which is used for the prevention of blood clots. Rivaroxaban takes effect through competitively inhibiting free and clot bound factor Xa, which is needed to activate prothrombin (factor II) to thrombin (factor IIa). The later is a serine protease that is required to activate fibrinogen to fibrin, which is the loose meshwork that completes the clotting process.
Each XARELTO tablet contains 10 mg, 15 mg, or 20 mg of rivaroxaban. The inactive ingredients of XARELTO are: croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. Additionally, the proprietary film coating mixture used for XARELTO 10 mg tablets is Opadry(R) Pink and for XARELTO 15 mg tablets is Opadry(R) Red, both containing ferric oxide red, hypromellose, polyethylene glycol 3350, and titanium dioxide, and for XARELTO 20 mg tablets is Opadry(R) II Dark Red, containing ferric oxide red, polyethylene glycol 3350, polyvinyl alcohol (partially hydrolyzed), talc, and titanium dioxide.
Indications and Usage
Rivaroxaban(366789-02-8) is an antithrombotic drug and was developed in a collaboration between the German Bayer Pharmaceuticals and American Johnson company. It is different from the traditional antithrombotic drug heparin in that Rivaroxaban does not need the participation of antithrombin III and can directly antagonize free and bound Xa factors. Heparin requires the effects of antithrombin III, and has no effect on Xa factors in the prothrombin complex. Rivaroxaban is the first oral direct Xa factor inhibitor in the whole world, and it can selectively and competitively inhibit free and bound Da factors as well as prothrombin activity. It uses a dose-dependent method to extend the partial thromboplastin time (PTT) and activated partial thromboplastin time (aPTT) to extend clotting time and lower thrombin genesis. Rivaroxaban has the characteristics of high bioavailability, wide range of target diseases, stable dose-effect relationship, easy oral intake, and low bleeding risk.
Rivaroxaban can also prevent and treat venous thrombosis. It is mainly used clinically to prevent deep venous thrombosis (DVT) and pulmonary embolism (PE) in adult patients following hip and knee replacement surgery. It is also used to prevent patients with nonvalvular atrial fibrillation from cerebral apoplexy and noncentral nervous system embolism, and it can lower the recurrence risk of coronary syndromes.
Mechanism of Action
Rivaroxaban(366789-02-8) is a selective inhibitor of FXa. It does not require a cofactor (such as Anti-thrombin III) for activity. Rivaroxaban inhibits free FXa and prothrombinase activity. Rivaroxaban has no direct effect on platelet aggregation, but indirectly inhibits platelet aggregation induced by thrombin. By inhibiting FXa, rivaroxaban decreases thrombin generation.
What is XARELTO?
XARELTO (Rivaroxaban,366789-02-8) is a prescription medicine used to:
- reduce the risk of stroke and blood clots in people who have a medical condition called atrial fibrillation. With atrial fibrillation, part of the heart does not beat the way it should. This can lead to the formation of blood clots, which can travel to the brain, causing a stroke, or to other parts of the body.
- treat blood clots in the veins of your legs (deep vein thrombosis) or lungs (pulmonary embolism) and reduce the risk of them occurring again
- reduce the risk of forming a blood clot in the legs and lungs of people who have just had hip or knee replacement surgery It is not known if XARELTO is safe and effective in children.
- It is not known if XARELTO is safe and effective in children.
Uses
Rivaroxaban(366789-02-8) is recommended as an option for treating pulmonary embolism and preventing recurrent deep vein thrombosis and pulmonary embolism in adults.
- Rivaroxaban (Xarelto, Bayer) is indicated for the 'treatment of deep vein thrombosis and pulmonary embolism, and prevention of recurrent deep vein thrombosis and pulmonary embolism in adults'. For the initial treatment of acute pulmonary embolism, the recommended dosage of rivaroxaban is 15 mg twice daily for the first 21 days followed by 20 mg once daily for continued treatment and prevention of recurrent venous thromboembolism.
- Non-Valvular Atrial Fibrillation (NVAF)* to prevent stroke & systemic embolism.
- Acute VTE treatment & prevention of recurrent VTE [for deep vein thrombosis (DVT) and pulmonary embolism (PE)].
- Prevention of venous thromboembolic events (VTE) in elective total hip or knee replacement surgery (THR, TKR).
important information about XARELTO
For people taking XARELTO for atrial fibrillation:
People with atrial fibrillation (an irregular heart beat) are at an increased risk of forming a blood clot in the heart, which can travel to the brain, causing a stroke, or to other parts of the body. XARELTO lowers your chance of having a stroke by helping to prevent clots from forming. If you stop taking XARELTO, you may have increased risk of forming a clot in your blood.
XARELTO can cause bleeding which can be serious, and rarely may lead to death. This is because XARELTO is a blood thinner medicine that reduces blood clotting. While you take XARELTO you are likely to bruise more easily and it may take longer for bleeding to stop.
You may have a higher risk of bleeding if you take XARELTO and take other medicines that increase your risk of bleeding, including:
- aspirin or aspirin containing products
- non-steroidal anti-inflammatory drugs (NSAIDs)
- warfarin sodium (Coumadin®, Jantoven®)
- any medicine that contains heparin
- clopidogrel (Plavix®)
- selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs)
- other medicines to prevent or treat blood clots
Carcinogenesis, Mutagenesis, Impairment of Fertility
Rivaroxaban(366789-02-8) was not carcinogenic when administered by oral gavage to mice or rats for up to 2 years. The systemic exposures (AUCs) of unbound rivaroxaban in male and female mice at the highest dose tested (60 mg/kg/day) were 1- and 2-times, respectively, the human exposure of unbound drug at the human dose of 20 mg/day. Systemic exposures of unbound drug in male and female rats at the highest dose tested (60 mg/kg/day) were 2- and 4-times, respectively, the human exposure.
Rivaroxaban was not mutagenic in bacteria (Ames-Test) or clastogenic in V79 Chinese hamster lung cells in vitro or in the mouse micronucleus test in vivo.
No impairment of fertility was observed in male or female rats when given up to 200 mg/kg/day of rivaroxaban orally. This dose resulted in exposure levels, based on the unbound AUC, at least 13 times the exposure in humans given 20 mg rivaroxaban daily.
Adverse Reactions
Bleeding events (may be serious or fatal), back pain, wound secretion, pruritus, pain in extremity, abdominal pain, blister.
Description
Rivaroxaban is an orally active, direct inhibitor of Factor Xa (Ki = 0.4 nM), which is a crucial component of the intrinsic and extrinsic pathways of the blood coagulation cascade. It demonstrates >10,000-fold greater selectivity for Factor Xa compared to other related serine proteases (thrombin, trypsin, plasmin, FVIIa, FIXa, FXIa, urokinase, and activated protein C). In various animal arterial and venous thrombosis models, rivaroxaban is reported to inhibit thrombin formation without prolonging bleeding time and has been approved for clinical use as an anticoagulant in the prevention of stroke and the treatment of venous thromboembolisms.
Chemical Properties
White Solid.
Rivaroxaban has limited pH-independent solubility in aqueous medium (5–7 mg/L; pH 1–9), but is, for instance, slightly soluble in polyethylene glycol 400 (2,431 mg/L). Using a validated Caco-2 cell assay, the apparent permeability values of the rivaroxaban molecule at concentrations of 1–100 μM were approximately 8 × 10?6 cm/s. With a log P value (octanol/water partition) coefficient of 1.5, rivaroxaban exhibits moderate lipophilicity, reflected in its low-to-moderate affinity to peripheral tissues[1].
IUPAC: 5-chloro-N-[[(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl]methyl]thiophene-2-carboxamide
Originator
Bayer (Germany)
Uses
Rivaroxaban is a novel antithrombotic agent. It is a novel, oral, selective direct inhibitor of factor Xa developed by Bayer Healthcare. It has been approved by the EMEA and FDA for the prevention ofvenous thromboembolism in adult patients after total hip replacement or total kneereplacement surgery.
Definition
ChEBI: Rivaroxaban is a monocarboxylic acid amide obtained by formal condensation of the carboxy group of 5-chlorothiophene-2-carboxylic acid with the amino group of 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl}morpholin-3-one. An anticoagulant used for prophylaxis of venous thromboembolism in patients with knee or hip replacement surgery. It has a role as an anticoagulant and an EC 3.4.21.6 (coagulation factor Xa) inhibitor. It is a member of thiophenes, an organochlorine compound, an oxazolidinone, a member of morpholines, a lactam, an aromatic amide and a monocarboxylic acid amide.
brand name
Xarelto
Pharmacokinetics
Rivaroxaban was absorbed rapidly with maximum plasma concentrations (Cmax) being reached 2–4 h after a single dose (1.25–80 mg) and multiple doses (up to 30 mg twice daily). Rivaroxaban did not accumulate to a relevant extent after multiple dosing. Data from phase I studies in healthy subjects showed that absorption is almost complete (oral bioavailability approached 80–100 %) for the 10 mg tablet dose, irrespective of fasting or fed conditions[3]. When administered orally with food, AUC and Cmax values were similar for whole and crushed rivaroxaban 20 mg tablets, whereas a crushed tablet suspended in water, administered using a nasogastric tube and followed by a liquid meal, gave similar AUC values but an 18 % reduction in Cmax.
Clinical Use
Factor Xa inhibitor:
Prevention of venous thromboembolism in adult
patients undergoing elective hip or knee replacement
surgery
Treatment of DVT or PE
Prophylaxis of stroke in AF
Prophylaxis of atherothrombotic events in ACS
Side effects
Regarding safety, there was no statistical difference in the incidence of major postoperative bleeding between any of the rivaroxaban dose groups and enoxaparin although there did appear to be a dose dependency in the rivaroxaban set. In addition to bleeding and subsequent posthemorrhagic anemia, presenting as weakness, paleness, asthenia, dizziness, headache, or unexplained swelling, other common adverse events included nausea, increased GGT, and an increase in transglutaminase. Owing to its mechanism of action, there is a bleeding risk, so the drug is contraindicated in patients with clinically active bleeding. Rivaroxaban is also contraindicated in pregnant and breast-feeding women and in patients with hepatic disease associated with coagulopathy and clinically relevant bleeding risk.
Synthesis
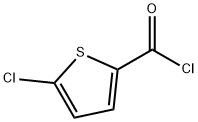
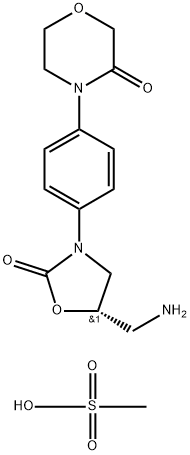
To date, several methods have been reported for the synthesis of rivaroxaban. Most of them share the use of 5-S-hydroxymethyl or 5-S-aminomethyl oxazolidinones (2and 3 respectively) as key intermediates. Condensation of 3-morpholinone with 4-fluoronitrobenzene followed by catalytic hydrogenation provides N-(p-aminophenyl)morpholinone for subsequent reaction with (S)-2-(phthalimidomethyl)oxirane. With establishment of the aminoalcohol adduct, cyclization with 1,1′-carbonyldiimidazole generates the central oxazolidinone. Deprotection and acylation with 5-chlorothiophene-2-carbonyl chloride affords rivaroxaban.
An Improved Synthesis of Rivaroxaban
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: increased risk of haemorrhage with IV
diclofenac and ketorolac - avoid.
Antibacterials: concentration reduced by rifampicin.
Anticoagulants: increased risk of haemorrhage with
other anticoagulants - avoid.
Antidepressants: concentration possibly reduced by
St John's wort.
Antiepileptics: concentration possibly reduced
by carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone.
Antifungals: concentration increased by ketoconazole
- avoid; avoid with itraconazole, posaconazole and
voriconazole.
Antivirals: avoid with atazanavir, darunavir,
fosamprenavir, indinavir, lopinavir, saquinavir and
tipranavir; avoid with lopinavir; concentration
increased by ritonavir - avoid.
Cobicistat: possibly enhanced effect with cobicistat
- avoid.
Metabolism
Metabolised by the cytochrome P450 isoenzymes CYP3A4 and CYP2J2 and by other mechanisms. About two-thirds of an oral dose is metabolised, with the metabolites excreted equally in the urine and faeces; the remaining third is excreted unchanged in the urine, mainly by active renal secretion.
Mode of action
Rivaroxaban is a direct, specific Factor Xa inhibitor. In vitro kinetic studies showed that the inhibition of human Factor Xa by rivaroxaban was competitive [inhibition constant (K i) 0.4 ± 0.02 nM] with 10,000-fold greater selectivity than for other serine proteases—it does not inhibit related serine proteases at concentrations up to 20 μM. This compound potently inhibited prothrombinase activity [inhibitory concentration 50 % (IC50) 2.1 ± 0.4 nM], and clot-associated Factor Xa activity (IC50 75 nM). In human plasma, rivaroxaban inhibited endogenous Factor Xa activity in a concentration-dependent manner with an IC50 of 21 ± 1 nM [19]. In an ex vivo study, rivaroxaban at a single dose of 5 mg and 30 mg reduced collagen-induced endogenous thrombin potential in human plasma by approximately 80 and 90 %, respectively, and tissue factor-induced endogenous thrombin potential by approximately 40 and 65 %, respectively. In contrast to indirect Factor Xa inhibitors, rivaroxaban does not require any cofactor to exert its anticoagulant effect. In human plasma, rivaroxaban concentration-dependently inhibited thrombin generation and, thus, the amplification processes of coagulation. Thrombin generation was almost completely inhibited at therapeutically relevant concentrations (80–100 nM) of rivaroxaban. In addition, rivaroxaban increased the permeability and degradability of the whole blood clot, by decreasing thrombin generation. However, rivaroxaban does not inhibit the activity of pre-existing thrombin molecules[1-2].
References
[1] Mueck, W., et al. "Clinical Pharmacokinetic and Pharmacodynamic Profile of Rivaroxaban."Springer Open Choice 53(2014):1-16.
[2] Perzborn, E., et al. "In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct Factor Xa inhibitor." Journal of Thrombosis and Haemostasis 3.3(2005):514-521.
[3] Kubitza, Dagmar , et al. "Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct Factor Xa inhibitor—after multiple dosing in healthy male subjects." European Journal of Clinical Pharmacology 61.12(2005):873-80.
Rivaroxaban Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 | dominicguo@gk-bio.com | CHINA | 9414 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 | xie@china-sinoway.com | China | 988 | 58 |
| TAIZHOU YUXIN BIOTECHNOLOGY CO,.LTD | +86-576-88902229;+86-0576-88902229 +8613968687450 | yuxin@yuxchem.com | China | 167 | 58 |
| Hefei TianRui Pharmaceutical Chemical Co., Ltd. | +86-0551-68665055 +86-+86-18616906106 | sales@trywchem.com | China | 148 | 58 |
| Jinan Dexinjia Bio&Tech Co., Ltd | +86-0531-82375893 +86-15064117275 | sales@jndxj.com | China | 1267 | 58 |
| Jinan Jianfeng Chemical Co., Ltd | 0531-88110457; +8615562555968 | info@pharmachemm.com | China | 248 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5893 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Sigma Audley | +86-15937194204 +86-18126314766 | nova@sh-teruiop.com | China | 467 | 58 |
Related articles
- Rivaroxaban: A Comprehensive Overview of Its Synthesis, Composition, and Applications
- Rivaroxaban is an anticoagulant medication (blood thinner) used to treat and prevent blood clots.Specifically it is used to tr....
- Oct 28,2024
- Rivaroxaban: Uses, Dose, Mechanism of action and Side effects
- Rivaroxaban is an oral antithrombotic medicine usually sold in the form of tablets and suspension (liquid).
- Jun 7,2024
- Rivaroxaban
- Rivaroxaban (Xarelto?), an oral oxazolidinone-based anticoagulant, is a potent, selective direct inhibitor of factor Xa
- Jul 26,2022
View Lastest Price from Rivaroxaban manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-11-22 | Rivaroxaban
366789-02-8
|
US $0.00-0.00 / Kg/Drum | 1KG | 98%-102% | 100 KGS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-11-22 | Rivaroxaban
366789-02-8
|
US $0.00-0.00 / KG | 1KG | USP/EP WC+DMF D90 < 10um | 20 tons | Sinoway Industrial co., ltd. | |
 |
2024-11-22 | Rivaroxaban
366789-02-8
|
US $0.00 / kg | 1kg | 99%min | 20ton | Jinan Jianfeng Chemical Co., Ltd |
-

- Rivaroxaban
366789-02-8
- US $0.00-0.00 / Kg/Drum
- 98%-102%
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Rivaroxaban
366789-02-8
- US $0.00-0.00 / KG
- USP/EP WC+DMF D90 < 10um
- Sinoway Industrial co., ltd.
-

- Rivaroxaban
366789-02-8
- US $0.00 / kg
- 99%min
- Jinan Jianfeng Chemical Co., Ltd
366789-02-8(Rivaroxaban)Related Search:
1of4






