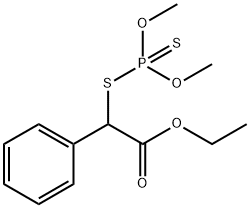
Phenthoate
- CAS No.
- 2597-03-7
- Chemical Name:
- Phenthoate
- Synonyms
- ELSAN;L 561;CIDIAL;S 2940;KAP(R);AIMSAN;Cydeal;FORSAN;Phendal;Cidemul
- CBNumber:
- CB9740624
- Molecular Formula:
- C12H17O4PS2
- Molecular Weight:
- 320.37
- MDL Number:
- MFCD00145099
- MOL File:
- 2597-03-7.mol
| Melting point | 17.5°C |
|---|---|
| Boiling point | 75°C |
| Density | approximate 1.23g/ml (20℃) |
| vapor pressure | 5.3×10-3 (40 °C) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), DMSO (Sparingly), Methanol (Slightly) |
| form | liquid |
| Water Solubility | 10 mg l-1(25 °C) |
| color | Colorless to light yellow |
| CAS DataBase Reference | 2597-03-7(CAS DataBase Reference) |
| EWG's Food Scores | 2 |
| FDA UNII | J96Q7F091K |
| NIST Chemistry Reference | Phenthoate(2597-03-7) |
| EPA Substance Registry System | Phenthoate (2597-03-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H312-H410 |
| Precautionary statements | P264-P270-P301+P312-P330-P501-P280-P302+P352-P312-P322-P363-P501-P273-P391-P501 |
| Hazard Codes | Xn,N |
| Risk Statements | 21/22-50/53 |
| Safety Statements | 22-36/37-60-61 |
| RIDADR | UN 2810 |
| RTECS | AI7875000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29309090 |
| Hazardous Substances Data | 2597-03-7(Hazardous Substances Data) |
Phenthoate Chemical Properties,Uses,Production
Description
Phenthoate is a colorless crystalline substance. Solubility in water is 10 mg/L (25 ?C). It is readily soluble in most organic solvents. Log Kow = 3.69. It is relatively stable in neutral and acidic aqueous media but decomposed under alkaline conditions.
Uses
Phenthoate is used to control sucking insects and caterpillars in a very wide range of crops. It is also used to control larval and adult mosquitoes
Uses
Phenthoate is an organothiophosphate insecticide used against lepidoptera and houseflies.
Definition
ChEBI: An organic thiophosphate that is ethyl mandelate in which the hydroxy group has been replaced by a (dimethoxyphosphorothioyl)sulfanediyl group.
Metabolic pathway
Studies which have measured the degradation of phenthoate in buffered aqueous solution have shown that it can be hydrolysed at the carboethoxy group, by cleavage of the P-S and C-S bonds and by demethylation in addition to oxidative desulfuration to the oxon. Reports on the metabolism of phenthoate in plants, insects and mammals have shown that the metabolic products can be largely explained by cleavage of these reactive groups. P-S and C-S bond cleavage is demonstrated by the formation of both O,O-dimethyl phosphorothioate and O,O-dimethyl phosphorodithioate. Metabolism in plants, insects and mammals is similar with many of the possible metabolites being identified in most species. Phenthoate carboxylic acid is a major metabolite except in plants where hydrolysis of the phosphorodithioate moiety predominates. In mammals, little or none of the activated metabolite, phenthoate oxon, can be detected, suggesting that the predominant metabolic route is degradative, wkuch may in part explain the favourable mammalian toxicity of the compound.
Metabolism
Phenthoate is degraded by hydrolysis of the carboethoxy moiety. Demethylation and the cleavage of P?S?C linkages are also important degradation routes. Oxidative desulfuration to the oxon followed by hydrolysis occurs in animals and plants.The major metabolites excreted in the urine and feces are demethyl phenthoate, demethyl phenthoate acid, demethyl phenthoate oxon, and O,O-dimethyl hydrogen phosphorodithioate and phosphorothioate. It is rapidly degraded in soils; DT50 was less than 1 d in both upland and submerged soil.
Toxicity evaluation
Acute oral LD50 for rats is 410 mg/kg. Inhalation LC50 (4 h) for rats is 3.17 mg/L air. NOEL (104 w) for dogs is 0.29 mg/kg daily. ADI is 3 μg/kg b.w.
Degradation
Phenthoate is stable in water under neutral and acid conditions but it
degrades under alkaline conditions (PM). Takade et al. (1976b) examined
the hydrolysis of [14C-phenyl]phenthoatein 0.01M phosphate buffer at
pH 6, 7 and 8 and [32P]phenthoatea t pH 8 (24.5 °C) and analysed the
products by TLC in comparison with non-radiolabelled authenticated
standards. The solutions were analysed for up to 28 days. Phenthoate was
fairly stable and degraded fastest at pH 8 with a DT50 of about 12
days. The major product of hydrolysis at all pH values was phenthoate
carboxylic acid (2), indicating that the carboethoxy moiety was the most
susceptible part of the molecule to hydrolysis. Significantly less
phenthoate carboxylic acid (2) was produced at pH 6. Quantities of desmethylphenthoate
oxon (3) were detected, particularly at pH 7 and 8, but
no phenthoate oxon (4), indicating that 3 may have been formed by the
oxidative desulfuration of desmethylphenthoate (5) which was a major
product, particularly at pH 6. The preponderance of demethylation at
lower pH values is found in most hydrolysis studies on organophosphorus
esters. Other minor products found were mandelic acid (6),
ethyl mandelate (7), bis-[a-(carboethoxy)benzyl] disulfide (8), a-(methylthio)
phenylacetic acid (9), desmethylphenthoate carboxylic acid (10)
and desmethylphenthoate oxon carboxylic acid (11) in addition to minor
products which could not be identified. The 32P study identified O,Odimethyl
phosphorothioate (12) and O,O-dimethyl phosphorodithioate
(13) as the hydrolytic products of the phosphorodithioate moiety. It was clear that although the major route of hydrolysis was due to cleavage
of the carboethoxy group, phenthoate could also be hydrolysed by three
additional mechanisms: (i) by cleavage of the P-O-Me linkage (demethylation)
to yield desmethylphenthoate (5); (ii) by P-S bond cleavage
to yield O,O-dimethyl phosphorothioate (12) and ethyl mercaptophenylacetate
(14) which then dimerised to the disulfide (8); (iii) by
C-S bond cleavage to O,O-dimethyl phosphorodithioate (13) and ethyl
mandelate (7). The occurrence of α-(methy1thio)phenylacetic acid (9) is
most likely explained by methylation of ethyl a-mercaptophenylacetate
(14) by phenthoate followed by hydrolysis (see Scheme 1). Reactions
involving each of these bonds give rise to a complex array of products,
many of which are detected in studies which have investigated the degradation
of phenthoate in biological systems.
Takade et al. (1976b) also investigated the photolysis of phenthoate
films spread on glass slides which were exposed to natural sunlight for up
to 42 hours. Much of the phenthoate was lost by volatilisation as well
as photolysis with a DT50 of about 15 hours and 90% disappeared after
42 hours. The principal photoproduct was phenthoate oxon (4) which
gradually increased to 20-30% after 35 hours exposure. Minor photoproducts
were desmethylphenthoate (5), mandelic acid (6), bis-[α-(carboethoxy
)benzyl] disulfide (S), bis-[α-carboxybenzyl] disulfide (15) and
O,O-dimethyl phosphorodithioate (13). The pathways for the hydrolytic
and photolytic decomposition of phenthoate are shown in Scheme 1.
Phenthoate Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
2597-03-7(Phenthoate)Related Search:
1of4