Cefetamet hydrochloride
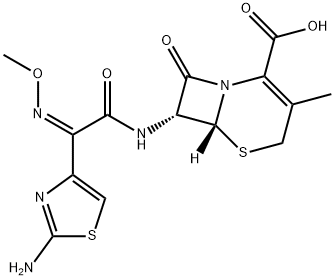
- CAS No.
- 65052-63-3
- Chemical Name:
- Cefetamet hydrochloride
- Synonyms
- Cefyl;Globocef;LY-97964;FR-13300;Ceftamet;LY-097964;CEFETAMET;Ro-15-8074;Ceftamet acid;CEFETAMET ACID
- CBNumber:
- CB0266851
- Molecular Formula:
- C14H15N5O5S2
- Molecular Weight:
- 397.43
- MOL File:
- 65052-63-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/18 11:31:16
| Melting point | >178°C (dec.) |
|---|---|
| Density | 1.83±0.1 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated, Sonicated) |
| pka | 3.11±0.50(Predicted) |
| form | Solid |
| color | White to Off-White |
| Stability | Hygroscopic |
| CAS DataBase Reference | 65052-63-3(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H335-H315-H319 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 |
| HS Code | 2941906000 |
Cefetamet hydrochloride Chemical Properties,Uses,Production
Uses
Cefetamet is a third-generation cephalosporin antibiotic and the active agent of Cefeamet pivoxil (C242780) after hydrolysis.
Antimicrobial activity
A semisynthetic cephalosporin formulated for oral use as the
prodrug ester, cefetamet pivoxyl. It is less active than cefaclor
and cefadroxil against Staph. aureus, but as active against
streptococci and more active against enterobacteria, H. influenzae
and N. gonorrhoeae, including β-lactamase-producing
strains. L. monocytogenes, C. difficile, Sten. maltophilia and Burk.
cepacia are all resistant. It is resistant to hydrolysis by common
plasmid-mediated enzymes.
The absolute bioavailability is about 50%. The plasma peak
is delayed by food. Binding to plasma protein is about 20%.
The volume of distribution approximates to the extracellular
water. It is excreted into urine with a half-life of 2–2.5 h,
principally in the glomerular filtrate. Elimination is linearly
related to creatinine clearance. Side effects and uses are similar
to those of other group 5 cephalosporins.
Cefetamet hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49392 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29825 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 8744 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418684 +8618949823763 | China | 25363 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 50000 | 58 | Inquiry |
| Shenzhen Excellent Biomedical Technology Co.,Ltd. | +86-0755-26050679 +86-15915472436 | China | 1031 | 58 | Inquiry |





