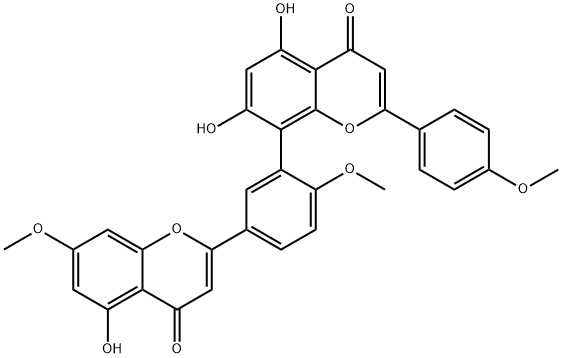
SCIADOPITYSIN
- CAS No.
- 521-34-6
- Chemical Name:
- SCIADOPITYSIN
- Synonyms
- Nsc 45108;SCIADOPITYSIN;Einecs 208-311-5;SCIADOPITYSIN(SH);4',4''',7-Trimethoxyamentoflavone;AMENTOFLAVONE-7,4',4'-TRIMETHYL ETHER;Amentoflavone-4',4",7-trimethyl Ether;AMENTOFLAVONE-7,4',4''-TRIMETHYL ETHER;AMENTOFLAVONE-7,4',4'''-TRIMETHYL ETHER;Sciadopitysin, 95%, from Ginkgo biloba L.
- CBNumber:
- CB0273404
- Molecular Formula:
- C33H24O10
- Molecular Weight:
- 580.54
- MOL File:
- 521-34-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/25 18:00:56
| Melting point | 296-298°C |
|---|---|
| Boiling point | 839.6±65.0 °C(Predicted) |
| Density | 1.445±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Soluble in ethanol and methan |
| form | powder |
| pka | 6.16±0.40(Predicted) |
| color | Light yellow |
| LogP | 5.500 (est) |
| CAS DataBase Reference | 521-34-6 |
SCIADOPITYSIN Chemical Properties,Uses,Production
Description
Sciadopitysin is a biflavonoid originally isolated from G. biloba and has diverse biological activities. It reduces cytotoxicity induced by amyloid-β (1-42) (Aβ42; ) in PC12 cells (EC50 = 9.84 μM). Sciadopitysin (0.1-10 μM) decreases methylglyoxal-induced insulin secretion, production of reactive oxygen species (ROS), cardiolipin peroxidation, and cytotoxicity in RIN-m5F pancreatic β-cells. It inhibits P-glycoprotein (P-gp; IC50 = 53.42 μM) and increases cellular toxicity of paraquat and paclitaxel in MDR1-MDCKII cells. Sciadopitysin inhibits RANKL-induced mRNA expression of the osteoclast-specific genes CTSK, TRAP, and MMP-9, activation of NF-κB, and osteoclastogenesis in a mouse model of LPS-induced bone loss.
Definition
ChEBI: A biflavonoid that is a 7, 4', 4'''-trimethyl ether derivative of amentoflavone.
SCIADOPITYSIN Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Chengdu Greenpure Biopharma CO.,Ltd | 18283602253; +8618283602253 | China | 952 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29898 | 58 | Inquiry |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | CHINA | 1824 | 58 | Inquiry |
| Chengdu Biopurify Phytochemicals Ltd. | +86-28-82633860 +86-18080483897 | China | 3424 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 | China | 4088 | 58 | Inquiry |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | China | 11616 | 58 | Inquiry |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | China | 10326 | 58 | Inquiry |
| Labnetwork lnc. | +86-27-50766799 +8618062016861 | China | 19994 | 58 | Inquiry |
521-34-6(SCIADOPITYSIN)Related Search:
1of4
chevron_right




