Valganciclovir hydrochloride
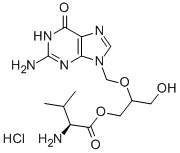
- CAS No.
- 175865-59-5
- Chemical Name:
- Valganciclovir hydrochloride
- Synonyms
- VALGANCICLOVIR HCL;Valcyte;Valcyt;Rs 079070-194;25MG/100MG/1KG;Unii-4p3T9qf9nz;Ro 107-9070/194;Valgancyclovir HCL USP;Valganciclovir hydrochlorid;Valganclicovir hydrochlcnde
- CBNumber:
- CB0380410
- Molecular Formula:
- C14H23ClN6O5
- Molecular Weight:
- 390.82
- MOL File:
- 175865-59-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 162-164°C |
|---|---|
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | H2O: ≥8mg/mL |
| form | powder |
| color | white to tan |
| Stability | Hygroscopic |
| InChIKey | ZORWARFPXPVJLW-MTFPJWTKSA-N |
| CAS DataBase Reference | 175865-59-5 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H312-H332 | |||||||||
| Precautionary statements | P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P330-P363-P501 | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 2933595960 | |||||||||
| NFPA 704 |
|
Valganciclovir hydrochloride price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SML0191 | Valganciclovir hydrochloride hydrate ≥98% (HPLC) | 175865-59-5 | 10MG | ₹11288.7 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SML0191 | Valganciclovir hydrochloride hydrate ≥98% (HPLC) | 175865-59-5 | 50MG | ₹45609.9 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1626 | Valganciclovir Hydrochloride Pharmaceutical Secondary Standard; Certified Reference Material | 175865-59-5 | 1G | ₹15966.88 | 2022-06-14 | Buy |
| TCI Chemicals (India) | V0158 | Valganciclovir Hydrochloride | 175865-59-5 | 250MG | ₹2900 | 2022-05-26 | Buy |
| TCI Chemicals (India) | V0158 | Valganciclovir Hydrochloride | 175865-59-5 | 1G | ₹6600 | 2022-05-26 | Buy |
Valganciclovir hydrochloride Chemical Properties,Uses,Production
Description
Valganciclovir hydrochloride, a prodrug of the antiviral ganciclovir, was launched in the US for the oral treatment of cytemegalovirus (CMV) retinitis, a sight-threatening complication in patients with AIDS. This L-Valyl ester prodrug can be prepared in three steps from the nucleoside analog ganciclovir by trimethylsilyl-protection of the amino group, coupling with N-benzyloxycarbonyl-L-valine-N-carboxyanhydride, hydrolysis with hydrochloric acid and hydrogenolysis of the Cbz-protecting group. Valganciclovir is well absorbed and rapidly hydrolyzed to ganciclovir by intracellular esterases in the intestinal mucosal cells and by hepatic esterases. Unlike ganciclovir, valganciclovir was demonstrated to be actively transported by the intestinal peptide transporter PEPT1 in Caco-2 cells. As a consequence, its absolute bioavailability in human was 10-fold higher compared to ganciclovir (6%). In clinical trials, it was shown that a twice-daily 900 mg dose of valganciclovir resulted in similar systemic ganciclovir exposure to 5 mg/kg twice-daily intravenous injection of ganciclovir. Valganciclovir concentrations could not be quantified in most patients within three to four hours. In a randomized non-blind phase III clinical trial, oral valganciclovir (900 mg twice daily for three weeks then 900 mg once daily for one week) was as effective as intravenous ganciclovir (5 mg/kg twice daily for three weeks then 5 mg/kg once daily). Oral treatment with valganciclovir avoided catheter-related infection that sometimes occurred with intravenous ganciclovir.
Chemical Properties
White Crystalline Solid
Uses
Valganciclovir hydrochloride hydrate may be used in HIV-related cell signaling studies.
General Description
Valganciclovir Hydrochloride is the L-valyl ester of ganciclovir and an antiviral drug, widely used to treat cytomegalovirus infections.
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to pharmacopeia primary standards.
Side effects
Side effects of Valganciclovir hydrochloride include: leukopenia, neutropenia, anaemia, thrombocytopenia, pancytopenia, bone marrow failure and aplastic anaemia. Based on animal data and limited human data, Valganciclovir Oral Solution may temporarily or permanently inhibit spermatogenesis in males and inhibit fertility in females, which in turn may lead to birth defects in humans. It may also cause cancer in humans.
Valganciclovir hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| KARPSCHEM LABORATORIES | +91-7249203006 +91-7249203006 | Maharashtra, India | 786 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Everest Organics Limited | +91-4040028838 +91-9440409996 | Hyderabad, India | 61 | 58 | Inquiry |
| Sun Pharmaceutical Industries Ltd | +91-2242244224 +91-2243244324 | Maharashtra, India | 108 | 58 | Inquiry |
| HRV Global Life Sciences | +91-9820219686 +91-9820219686 | Telangana, India | 379 | 58 | Inquiry |
| Aurobindo Pharma Limited | +914066725000 | Telangana, India | 112 | 58 | Inquiry |
| Arene Lifesciences Limited | +91-9849847907 +91-8455241148 | Telangana, India | 55 | 58 | Inquiry |
| BDR Pharmaceuticals International Pvt Ltd | +91-2240560560 +91-7718884418 | Maharashtra, India | 206 | 58 | Inquiry |
| Bakul Pharma Private Limited | +91-2261697900 +91-2261697900 | Maharashtra, India | 66 | 58 | Inquiry |
| Aspen Biopharma Labs Pvt Ltd | +91-9248058660 +91-9248058662 | Telangana, India | 234 | 58 | Inquiry |





