Valaciclovir
- CAS No.
- 124832-26-4
- Chemical Name:
- Valaciclovir
- Synonyms
- VALACYCLOVIR;VALACICLOVIR HYDROCHLORIDE;VALACICLOVIR HCL;256U87;VALACV;Valcivir;VALACICLOVIR;100MG/1G/1KG;Valacyclovir-d4;ValaciclovirBase
- CBNumber:
- CB2379191
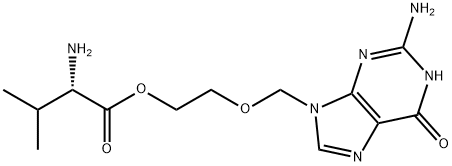
- Molecular Formula:
- C13H20N6O4
- Molecular Weight:
- 324.34
- MOL File:
- 124832-26-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/7/19 17:32:57
| Density | 1.55±0.1 g/cm3(Predicted) |
|---|---|
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | 9.34±0.20(Predicted) |
| EPA Substance Registry System | L-Valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]ethyl ester (124832-26-4) |
Valaciclovir Chemical Properties,Uses,Production
Chemical Properties
White Solid
Uses
The L-Valine ester prodrug of Acyclovir.
Valacyclovir (Valtrex) is the 1-valine ester (prodrug) of acyclovir that exhibits no activity until hydrolyzed in the intestinal wall or liver to acyclovir and its active metabolite. Its modified structure allows increased intestinal absorption and concomitant higher plasma levels of acyclovir. It demonstrates activity against HSV types 1 and 2, varicella-zoster virus, and cytomegalovirus. It exerts its effects by interfering with DNA synthesis through phosphorylation by viral thymidine kinase and subsequent inhibition of viral DNA polymerase, thereby inhibiting viral replication. Valtrex is indicated for the treatment of acute herpes zoster and recurrent genital herpes in immunocompetent adults. The most common side effects are headache, nausea, and vomiting.
Indications
Valacyclovir (Valtrex) is the 1-valine ester (prodrug) of acyclovir that exhibits
no activity until hydrolyzed in the intestinal wall or liver to acyclovir and its
active metabolite. Its modified structure allows increased intestinal absorption
and concomitant higher plasma levels of acyclovir. It demonstrates activity
against HSV types 1 and 2, varicella-zoster virus, and cytomegalovirus.
It exerts its effects by interfering with DNA synthesis through phosphorylation
by viral thymidine kinase and subsequent inhibition of viral DNA
polymerase, thereby inhibiting viral replication. Valtrex is indicated for the
treatment of acute herpes zoster and recurrent genital herpes in immunocompetent
adults. The most common side effects are headache, nausea, and
vomiting.
Valacyclovir is the only antiviral agent approved for herpes labialis,
for a 3-day course in the episodic treatment of recurrent genital herpes (2).
Valacyclovir is indicated for recurrent genital herpes and is administered at
500 mg twice daily for 3 days at the onset of prodromal symptoms or at the first
sign of infection.
Pharmacology
Valacyclovir (Valtrex), a valine ester prodrug of acyclovir, has a bioavailability three to five times that of acyclovir. This is due to its improved gastrointestinal absorption compared to acyclovir. Valacyclovir is rapidly and almost completely converted to acyclovir after oral administration, with levels comparable to those of intravenous acyclovir. This allows for twice-daily dosing, which may improve compliance and ultimate clinical efficacy. It is more costly than acyclovir.
Valaciclovir Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CHEMXTREE STANDARDS | +919872732255 | Punjab, India | 96 | 58 | Inquiry |
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| J S LABS | +91-7330612784 +91-7330612784 | Tamil Nadu, India | 160 | 58 | Inquiry |
| Medchem Labs | +91-7288889564 +91-7288889555 | Telangana, India | 25 | 58 | Inquiry |
| Rivashaa Agrotech Biopharma Pvt. Ltd. | +91-26463395 +91-7926462688 | Gujarat, India | 1615 | 58 | Inquiry |
| Amara Labs Pvt Ltd | +91-9553279972 +91-9100091565 | Hyderabad, India | 42 | 58 | Inquiry |
| Tivan Sciences Pvt Ltd | +91-9081499099 +91-9081499099 | Gujarat, India | 14 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| BDR Pharmaceuticals International Pvt Ltd | +91-2240560560 +91-7718884418 | Maharashtra, India | 206 | 58 | Inquiry |
| Exemed Pharmaceuticals (Oneiro Chemicals Pvt Ltd) | +91-9687684674 +91-9099061028 | Gujarat, India | 30 | 58 | Inquiry |
124832-26-4(Valaciclovir)Related Search:
1of4
chevron_right





