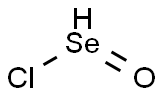
Selenium Oxychloride
- CAS No.
- 7791-23-3
- Chemical Name:
- Selenium Oxychloride
- Synonyms
- Selenyl chloride;SELENINYL CHLORIDE;seleninyldichloride;SELENIUM OXYCHLORIDE;seleniumchlorideoxide;seleniumoxydichloride;Selenium oxidichloride;SELENIUM(IV) OXYCHLORIDE;SELENIUM OXYCHLORIDE 99%;Selenium (Ⅳ) oxychloride
- CBNumber:
- CB0397694
- Molecular Formula:
- Cl2OSe
- Molecular Weight:
- 165.87
- MOL File:
- 7791-23-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/11/28 16:31:44
| Melting point | 9 °C |
|---|---|
| Boiling point | 180 °C(lit.) |
| Density | 2.43 g/mL at 25 °C(lit.) |
| refractive index | 1.651 |
| Flash point | 176.4°C |
| solubility | Miscible with chloroform, carbon disulphide and benzene. |
| form | Liquid |
| Specific Gravity | 2.42 |
| color | Yellow |
| Water Solubility | decomposed in H2O to HCl, selenious acid; miscible with CCl4, CHCl3, CS2, benzene, toluene [MER06] |
| Sensitive | Moisture Sensitive |
| Merck | 14,8435 |
| Exposure limits |
ACGIH: TWA 0.2 mg/m3 NIOSH: IDLH 1 mg/m3; TWA 0.2 mg/m3 |
| Dielectric constant | 46.0 |
| Stability | Stable, but reacts with water and moisture. Incompatible with water, potassium, phosphorus. |
| CAS DataBase Reference | 7791-23-3(CAS DataBase Reference) |
| EPA Substance Registry System | Selenium oxychloride (7791-23-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H331-H373-H410 | |||||||||
| Precautionary statements | P260-P264-P273-P301+P310-P304+P340+P311-P314 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 14-23/25-33-35-50/53 | |||||||||
| Safety Statements | 26-36/37/39-45-60-61-28-20/21 | |||||||||
| RIDADR | UN 2879 8/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | VS7000000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 28121099 | |||||||||
| Toxicity | LD50 s.c. in rabbits: 7 mg/kg (Wilber) | |||||||||
| NFPA 704 |
|
Selenium Oxychloride price More Price(2)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| ALFA India | ALF-068108-AC | Selenium dichloride oxide, 99% (metals basis) | 7791-23-3 | 25mL | ₹55038 | 2022-05-26 | Buy |
| ALFA India | ALF-068108-AB | Selenium dichloride oxide, 99% (metals basis) | 7791-23-3 | 10mL | ₹25815 | 2022-05-26 | Buy |
Selenium Oxychloride Chemical Properties,Uses,Production
Chemical Properties
Selenium oxychloride is a colorless to yellowish liquid. Fumes in air.
Physical properties
Pale yellow or colorless liquid; corrosive; refractive index 1.651 at 20°C; density 2.42 g/mL at 22°C; freezes at 8.5°C; boils at 176.4°C; decomposes at 176.4°C; decomposes in water forming hydrochloric acid and selenious acid; soluble in carbon disulfide, carbon tetrachloride, chloroform, benzene, and toluene.
Uses
selenium oxychloride is used as solvent.
Production Methods
Selenium oxychloride (SeOCl2) is a powerful solvent, chlorinating agent, and resin plasticizer used in the chemical industry.
General Description
A colorless to light-colored liquid. Insoluble in water and denser than water. Contact may severely irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Used to make other chemicals.
Air & Water Reactions
Fumes in air. Insoluble in water and denser than water. Decomposed in water or moist air to form hydrochloric acid and selenious acid [Merck 11th ed. 1989].
Reactivity Profile
Red phosphorus reacts in the cold with SELENIUM OXYCHLORIDE evolving light and heat; white phosphorus reacts explosively [Mellor 10:906 1946-47]. When potassium is brought into contact with SELENIUM OXYCHLORIDE in the cold, an explosion occurs [Mellor 10:908 1946-47]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. Powdered antimony ignites on contact with the chloride, [Mellor, 1947, Vol. 10, 906]. With metal oxides, i.e. silver oxide, light is evolved and heat sufficient to decompose the mixture, [Mellor, 1947, Vol. 10, 909].
Hazard
Extremely hazardous, toxic irritant, poison, severe vesicant.
Health Hazard
SELENIUM OXYCHLORIDE is very toxic and may cause death or permanent injury after very short exposures to small quantities. Inhalation of small quantities may be corrosive and irritating to the respiratory tract. It can burn and irritate the skin and eyes and cause burns when ingested. Long-term exposure to selenium compounds may be a cause of amyotrophic lateral sclerosis in humans. Populations at special risk include those with a history of dermatitis, chronic bronchitis, skin allergies, respiratory tract infections, liver or kidney disease, jaundice, or albuminuria. Women of child-bearing age are also considered at risk.
Fire Hazard
When SELENIUM OXYCHLORIDE is heated to decomposition, or in contact with acids or acid fumes, highly toxic chloride and selenium fumes are evolved. Hydrochloric acid and selenious acid are produced by reaction with water. Decomposed by water. Reacts violently with powdered antimony, red and white phosphorus, disilver oxide, lead oxides, and potassium. Avoid water, moist air.
Safety Profile
Poison by skin contact and subcutaneous routes. Human systemic effects by skin contact with very small amounts: primary irritant, corrosive. Explodes on contact with potassium, white phosphorus. Ignites on contact with antimony. Vigorous reaction with metal oxides (e.g., silver oxide, lead@) oxide, lead(Ⅳ) oxide, lead(II)(IV) oxide). When heated to decomposition it emits very toxic fumes of Cland Se. See also SELENIUM COMPOUNDS and CHLORIDES.
Potential Exposure
This material is used as a solvent for many substances, including metals and as a chlorinating agent; and resin plasticizer; as an ionizing solvent; for monochlorination of ketones.
Shipping
UN2879 Selenium oxychloride, Hazard class: 8; Labels: 8-Corrosive material, 6.1-Poisonous material.
Incompatibilities
Water and air reactive releasing strong acids. The aqueous solution is a strong acid and oxidizer.Reacts violently with bases, reducing agents, hydrides, powdered antimony, red, and white phosphorus, disilver oxide, lead oxide; powdered metals, and potassium. Note: Never pour water into this substance; when dissolving or diluting always add it slowly to the water
Selenium Oxychloride Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Maharashtra Organo Metallic Catalysts Pvt. Ltd. | 08048961322 | Mumbai, India | 27 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9003 | 58 | Inquiry |
| ABCR GmbH & CO. KG | 49 721 95061 0 | Germany | 6846 | 75 | Inquiry |
| Strem Chemicals, Inc. | (978) 462-3191 | United States | 4911 | 86 | Inquiry |
| Pfaltz & Bauer, Inc. | (800) 225-5172 | United States | 6830 | 72 | Inquiry |
| FUJIFILM Wako Pure Chemical Corporation | +81 6 6203 3741 | Japan | 6222 | 58 | Inquiry |
| Molekula Limited | 44-3302-000333 | United Kingdom | 6130 | 58 | Inquiry |
7791-23-3(Selenium Oxychloride)Related Search:
1of4
chevron_right




