CARVONE
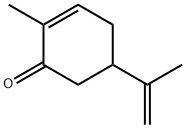
- CAS No.
- 99-49-0
- Chemical Name:
- CARVONE
- Synonyms
- DL-carvone;p-Mentha-6(1),8-dien-2-one;2-methyl-5-(1-methylethenyl)-2-Cyclohexen-1-one;5-Isopropenyl-2-methyl-2-cyclohexen-1-one;2-Methyl-5-(1-methylethenyl)-2-cyclohexene-1-one;2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)-;Karvon;Cavone;CARVONE;NSC 6275
- CBNumber:
- CB0773601
- Molecular Formula:
- C10H14O
- Molecular Weight:
- 150.22
- MOL File:
- 99-49-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/12/18 13:37:16
| Melting point | 230℃ |
|---|---|
| Boiling point | 232℃ (760.0 Torr) |
| Density | 0.963 g/cm3 (15℃) |
| FEMA | 2249 | CARVONE |
| refractive index | 1.710 |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Oil |
| color | Light Orange to Light Brown |
| Odor | at 100.00 %. minty licorice |
| Odor Type | minty |
| JECFA Number | 380 |
| Dielectric constant | 11.0(22℃) |
| Stability | Light Sensitive |
| LogP | 3.07 |
| CAS DataBase Reference | 99-49-0 |
| EPA Substance Registry System | 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)- (99-49-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H317 |
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501 |
| Hazardous Substances Data | 99-49-0(Hazardous Substances Data) |
CARVONE Chemical Properties,Uses,Production
Chemical Properties
Carvone occurs as (S)-(+)-
carvone ([α]18
D +64.3°,), (R)-(?)-carvone ([α]20
D ?62.5°),
or racemic carvone. The optical isomers differ considerably in their
sensory properties. They occur in high percentages in a number of essential oils.
(+)-Carvone is the main component of caraway oil (about 60%) and dill oil; (?)-
carvone occurs in spearmint oil at a concentration of 70–80%.
Both (+)- and (?)-carvone are used to flavor a number of foods and beverages.
(?)-Carvone is produced in much larger quantities and is mainly used in
oral hygiene products.
Physical properties
The carvones are colorless to slightly yellow liquids.(+)-Carvone has a herbaceous odor reminiscent of caraway and dill seeds, whereas (?)-carvone has a herbaceous odor reminiscent of spearmint. Depending on the reaction conditions, hydrogenation of carvone yields either carveol or dihydrocarvone, which are also used as flavor compounds. When treated with strong acids, carvone isomerizes to carvacrol.
Occurrence
The optically active and inactive forms have been reported among the constituents of about 70 essential oils. The dextro form is present in carvi, Antheum graveolens, Antheum sowa, Lippia carviodora, Mentha arvensis, etc. The levo form is present in Metha vifidis var. crispa, Mentha longifolia from South Africa, Eucalyptus globules and several mint species. The racemic form is present in ginger grass, Litsea gutalemaleusis, lavender and Artemisia ferganensis. Reported found in citrus oil and juice (lemon, lime, orange), celery seed, anise, clove, coriander seed, calamus, caraway herb and dill seed.
Uses
Carvone is useful for the treatment of various metabolic disorders and GI related disorders.
Preparation
In the past, (+)- and (?)-carvones were isolated by fractional distillation
of caraway oil and spearmint oil, respectively. However, these carvones are
now prepared synthetically, the preferred starting materials being (+)- and (?)-
limonenes, which are converted into the corresponding optically active carvones.
Since optical rotation is reversed in the process, (+)-limonene is the startingmaterial
for (?)-carvone.
Thepreferred industrialmethod of carvone synthesis utilizes the selective addition
of nitrosyl chloride to the endocyclic double bond of limonene. If a lower
aliphatic alcohol is used as solvent, limonene nitrosochloride is obtained in high
yield. It is converted into carvone oxime by elimination of hydrogen chloride in
the presence of a weak base. Acid hydrolysis in the presence of a hydroxylamine
acceptor, such as acetone, yields carvone.
An alternative process for the production of (?)-carvone has recently been commercialized.
Starting from (+)-limonene 1,2-epoxide, a regioselective rearrangement
of the epoxide leads to (?)-carveol (trans- :[2102-58-1]; cis- :[2102-59-2]).
Thereaction is effected by the use of a catalyst consisting of a combination of metal
salts and phenolic compounds.
(?)-Carveol is subsequently oxidized to (?)-carvone by anOppenauer oxidation
or by dehydrogenation in the presence of special catalysts.The reaction may also
be performed as a one-pot reaction.
Definition
A ketone derived from the terpene dipentene. It is optically active, occurring naturally in both d- and l-forms.
CARVONE Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6700 | 30 | Inquiry |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | China | 8808 | 58 | Inquiry |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | China | 12809 | 58 | Inquiry |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 | China | 18137 | 58 | Inquiry |
| Hebei Zhuanglai Chemical Trading Co Ltd | +86-16264648883 +86-16264648883 | China | 7245 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | China | 29730 | 60 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29859 | 58 | Inquiry |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 | China | 2930 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 | China | 3978 | 58 | Inquiry |





