Nitrosyl chloride
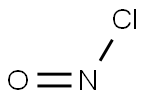
- CAS No.
- 2696-92-6
- Chemical Name:
- Nitrosyl chloride
- Synonyms
- Nitrosylchlorid;Nitroso chloride;Nitrosyl chloride;Nitrogen oxychloride;Nitrosyl chloride ((NO)Cl);Nitrosyl chloride ISO 9001:2015 REACH
- CBNumber:
- CB5851670
- Molecular Formula:
- ClNO
- Molecular Weight:
- 65.46
- MOL File:
- 2696-92-6.mol
- Modify Date:
- 2023/11/28 16:31:44
| Melting point | -59.6°C |
|---|---|
| Boiling point | -5 |
| Density | 1.417 |
| vapor density | 2.3 |
| solubility | reacts with H2O |
| form | yellow-red-brown gas |
| color | Orange-yellow gas condensing to a deep red liquid |
| Odor | irritating odor |
| Water Solubility | decomposes in H2O; soluble fuming H2SO4 [HAW93] |
| Dielectric constant | 18.0(-12℃) |
| LogP | 0.351 (est) |
| EPA Substance Registry System | Nitrosyl chloride (2696-92-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS03,GHS05,GHS04,GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H330-H280-H314-H270 |
| Precautionary statements | P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501-P220-P244-P370+P376-P403-P410+P403-P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501 |
| Hazard Codes | C,T |
| RIDADR | 1069 |
| Hazard Note | Toxic/Corrosive |
| HazardClass | 2.3 |
Nitrosyl chloride Chemical Properties,Uses,Production
Chemical Properties
Yellow-red liquid or yellow gas.Decomposed by water; dissociates into nitric oxide and chlorine on heating; soluble in fuming sulfuric acid. Nonflammable.
Physical properties
Yellow gas; heavier than air, density 2.3 (air=1); gas density 2.872 g/L; liquefies at -5.55°C; liquid density 1.273 g/mL; freezes at -59.4°C; critical temperature 167°C; reacts with water; soluble in fuming sulfuric acid.
Uses
Synthetic detergents, catalyst, intermediate.
Preparation
Nitrosyl chloride can be prepared by the reaction of nitric oxide with chlorine: 2NO + Cl2 → 2ClNO
Also, nitrosyl chloride is produced by the action of chlorine on sodium nitrate; or by the reaction of nitrosyl sulfuric acid with hydrochloric acid: NaNO3 + Cl2 → ClNO + NaClO2 ONHSO4 + HCl → ClNO + H2 SO4
Nitrosyl chloride also is obtained as a byproduct in the manufacture of potassium nitrate from potassium chloride and nitric acid:In the above preparative method, nitrosyl chloride must be separated from nitric acid; otherwise, in the presence of pure and excess nitric acid, it can decompose to nitrogen dioxide and chlorine:2ClNO + HNO3 → 6NO2 + Cl2 +2H2O
Also, nitrosyl chloride can be synthesized from its elements by heating nitrogen, oxygen and chlorine gas at 400°C: N2 + O2 + Cl2 → 2ClNO
Faraday obtained nitrosyl chloride by dissolving palladium in a mixture of hydrochloric and nitric acids (Faraday, M. Trans. Roy. Soc. (London), Vol. 136, pp. 48, 1846): Pd + HNO3 + 3HCl → PdCl2 + ClNO + 2H2O.
Definition
One of the oxidizing agents in aqua regia.
General Description
A yellow to yellowish red gas. Liquefies at -5.5°C. Very toxic by inhalation. Noncombustible, but accelerates burning of combustible material. Decomposed by water to form corrosive hydrochloric acid. Vapors heavier than air. Prolonged exposure to fire or heat can cause containers to rupture violently and rocket .
Air & Water Reactions
Dissolves into and reacts with moisture in the air or with water to form hydrochloric acid and toxic red oxides of nitrogen.
Reactivity Profile
Nitrosyl chloride is an oxidizing agent. Dissociates into nitric oxide and chlorine on heating. Gives an explosive reaction when sealed in a tube with a residue of acetone and in the presence of platinum catalyst [Chem. Eng. News 35(43):60. 1967]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Hazard
Strong irritant, especially to lungs and mucous membranes.
Health Hazard
Gas is highly toxic. Inhalation causes severe irritation of respiratory tract and damage to mucous membranes. Delayed effects, which include severe pulmonary edema, may not be apparent for several hours.
Fire Hazard
Special Hazards of Combustion Products: Very toxic gases are generated when heated
Safety Profile
Poison by inhalation and ingestion. A corrosive irritant to skin, eyes, and mucous membranes. Inhalation may cause pulmonary edema and hemorrhage. Potentially explosive reaction with acetone + platinum. Mixtures with hydrogen + oxygen igmte spontaneously. When heated to decomposition it emits very toxic fumes of Cland NOx.
Potential Exposure
A nitrocompound used as a reagent, catalyst, bleaching agent, and intermediate for making other chemicals.
Shipping
UN1069 Nitrosyl chloride, Hazard Class: 2.3; Labels 2.3-Poisonous gas, 8-Corrosive material; Inhalation Hazard Zone C
Purification Methods
It is an orange gas with a suffocating odour. It has been fractionally distilled at atmospheric pressure in an all-glass, low-temperature still, taking the fraction boiling at -4o and storing it in sealed tubes. Alternatively the gas is dried by CaCl2 and passed through H2SO4 when Cl2 passes on, but NOCl is absorbed to form nitrososulfuric acid (NO.HSO4) which on warming with NaCl evolves pure NOCl [Tilden J Chem Soc 27 630 1874.] It is decomposed by H2O and alkali, and forms compounds with metal chlorides e.g. FeCl3.NOCl. [Coleman Inorg Synth I 55 1939.]
Incompatibilities
Dissolves into and reacts with moisture in the air or with water forming hydrochloric acid and toxic red oxides of nitrogen. Nitrosyl chloride is a strong oxidizer. Violent reaction with strong acids, alkalis (e.g., sodium hydroxide), ammonia, amines, reducing agents, other strong oxidizers. Elevated temperature may cause explosive decomposition. Dissociates into nitric oxide and chlorine on heating. Corrosive to most metals. Attacks some plastics, rubber, and coatings.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Nitrosyl chloride Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 | China | 7353 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 | China | 2132 | 58 | Inquiry |
| tonsooh | 027-83238443 13343438265 | China | 4992 | 58 | Inquiry |
| Isotope (Xiamen) Industry and Trade Co., Ltd | 0510-051082803581 15903302207 | China | 229 | 58 | Inquiry |
| Wuhan canos Technology Co., Ltd | 153-37111468; 15337111468 | China | 4923 | 58 | Inquiry |
| Hubei coward Chemical Co., Ltd | 13125172151; 13125172151 | China | 5001 | 58 | Inquiry |
| Hubei Taihechang Biotechnology Co., Ltd | 18607169902 18607169902 | China | 2425 | 58 | Inquiry |
| Wuhan Xinzhongke Chemical Technology Co., Ltd | 13628625909 | China | 2487 | 58 | Inquiry |
| Hubeicowardchemical | 027-83295551 13317178958 | China | 4874 | 58 | Inquiry |
| Shaanxi Xihua Chemical Industry Co., Ltd | 029-81122149 13992773979 | China | 6288 | 58 | Inquiry |
2696-92-6(Nitrosyl chloride)Related Search:
1of2
chevron_right




