Copper dinitrate
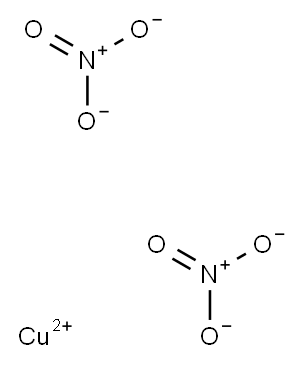
- CAS No.
- 3251-23-8
- Chemical Name:
- Copper dinitrate
- Synonyms
- CU(NO3)2;COPPER NITRATE;Cupric nitrate;claycop;COPPER (II) NITRATE;cupricdinitrate;Copper dinitrate;copper(2+)nitrate;Kupfer(II)-nitrat;Copperbis(nitrate)
- CBNumber:
- CB1219900
- Molecular Formula:
- CuN2O6
- Molecular Weight:
- 187.56
- MOL File:
- 3251-23-8.mol
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 115°C |
|---|---|
| Density | 1.00 g/mL at 20 °C |
| vapor pressure | 0Pa at 25℃ |
| solubility | soluble in dioxane; reacts with ethyl ether |
| form | blue-green orthorhombic crystals |
| color | blue-green orthorhombic crystals, crystalline; hygroscopic |
| Water Solubility | Soluble |
| Merck | 13,2671 |
| Stability | Stable. Oxidant. Incompatible with combustible materials. |
| Surface tension | 73.2mN/m at 1.3g/L and 20.2℃ |
| CAS DataBase Reference | 3251-23-8(CAS DataBase Reference) |
| EPA Substance Registry System | Cupric nitrate (3251-23-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS03, GHS05, GHS07, GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H272-H315-H318-H335-H410 | |||||||||
| Precautionary statements | P220-P261-P273-P280-P305+P351+P338-P501 | |||||||||
| Hazard Codes | O,C,Xi,N,Xn | |||||||||
| Risk Statements | 8-20/21/22-34-22-36/38-20-45-51/53-41-38-50/53-37/38-52/53 | |||||||||
| Safety Statements | 53-17-26-36/37/39-45-36-61-39 | |||||||||
| RIDADR | UN 3085 5.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | II | |||||||||
| Hazardous Substances Data | 3251-23-8(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Copper dinitrate Chemical Properties,Uses,Production
Chemical Properties
Cupric nitrate is a Blue crystalline solid.
Uses
Light-sensitive papers; analytical reagent; mordant in textile dyeing; nitrating agent; insecticide for vines; coloring copper black; electroplating; production of burnished effect on iron; paints; varnishes, enamels; pharmaceutical preparations; catalyst.
Definition
ChEBI: An inorganic nitrate salt having copper(2+) as the couterion.
General Description
Obtained as a trihydrate and as a hexahydrate. Both are blue crystalline solids. Used in medicine, as an insecticide, in chemical analysis, in making light sensitive papers. Toxic oxides of nitrogen are produced in fires involving Copper dinitrate.
Air & Water Reactions
Deliquescent. Water soluble.
Reactivity Profile
Mixtures of Copper dinitrate with alkyl esters may explode, owing to the formation of alkyl nitrates. Mixtures with phosphorus, tin(II) chloride or other reducing agents may react explosively [Bretherick, 1979 p. 108-109]. A finely divided mixture of potassium ferrocyanide and Copper dinitrate exploded when dried at 220°C [Chem. Abst. 77:1343 (1972)]. Noncombustible, but Copper dinitrate will accelerate the burning of combustible materials. If large quantities of the material are involved in a fire or the material is finely divided, an explosion may result. Prolonged exposure to fire or heat may result in an explosion.
Hazard
Oxidizer, causes violent combustion or explosion with organic materials.
Health Hazard
Inhalation causes irritation of throat and lungs. Ingestion of large amounts causes violent vomiting and purging, intense pain, collapse, coma, convulsions, and paralysis. Solutions irritate eyes; contact with solid causes severe eye surface injury and skin irritation.
Safety Profile
Moderately toxic by ingestion. A severe eye and skin irritant. Potentially explosive reaction above 22OOC with ammonium or potassium hexacyanoferrate(I1). Reaction with ammonia + potassium amide gives explosive product. Violent reaction with acetic anhydride. May ignite on prolonged contact with paper. Concentrated solutions may ipte in contact with tin or aluminum foil. Used as a fungicide, herbicide, and as a catalyst component in solid rocket fuel. When heated to decomposition it emits toxic fumes of NOx. See also COPPER COMPOUNDS and NITRATES.
Potential Exposure
Cupric nitrate is used as an insecticide, in paint, varnish, enamel, and in wood preservatives. Metal compounds are often used in “hot” operations in the work-place. These may include, but are not limited to, welding, brazing, soldering, plating, cutting, and metallizing. At the high temperatures reached in these operations, metals often form metal fumes which have different health effects and exposure standards than the original metal compound and require specialized controls.
Shipping
UN1479 Oxidizing solid, n.o.s., Hazard Class: 5.1; Labels: 5.1-Oxidizer, Technical Name Required.
Purification Methods
Crystallise it from weak aqueous HNO3 (0.5mL/g) by cooling from room temperature. The anhydrous salt can be prepared by dissolving copper metal in a 1:1 mixture of liquid NO2 and ethyl acetate and purified by sublimation [Evans et al. J Chem Soc, Faraday Trans 1 75 1023 1979]. The hexahydrate dehydrates to the trihydrate at 26o, and the anhydrous salt sublimes between 150 and 225o, but melts at 255-256o and is deliquescent.
Incompatibilities
A strong oxidizer. Aqueous solution is acidic; incompatible with bases. Violent reaction with potassium hexacyanoferrate; ammonia and potassium amide mixtures; acetic anhydrides, cyanides, ethers. Forms explosive materials with nitromethanes, sodium hypobromite; acetylene; chemically active metals, such as potassium, sodium, etc. May ignite on contact with aluminum foil or tin. Risk of spontaneous combustion with combustibles (wood, cloth, etc.) organics, or reducing agents and readily oxidizable materials. Attacks metals in the presence of moisture.
Waste Disposal
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill. Add slowly to water; stir in excess soda ash. Let stand, then neutralize. Decant solution and flush to sewer; landfill sludge
Copper dinitrate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| QUALIKEMS FINE CHEM PVT LTD | +911123618475 | New Delhi, India | 51 | 58 | Inquiry |
| Yogi Dye Chem Industries | +91-9821098577 +91-9821098577 | Mumbai, India | 124 | 58 | Inquiry |
| Evans Fine Chem | +91-9821340302 +91-9821340302 | Maharashtra, India | 286 | 58 | Inquiry |
| Hemal Impex | +91-2646220650 +91-9824111508 | Maharashtra, India | 14 | 58 | Inquiry |
| Nithyasri Chemicals (APURVA CHEMICALS) | +91-2512692130 +91-9323652199 | Maharashtra, India | 75 | 58 | Inquiry |
| Eureka Chemicals | +91-2222074176 +91-2222074206 | Maharashtra, India | 11 | 58 | Inquiry |
| Cosmic Chemicals | +91-8806467677 +91-9324619871 | Maharashtra, India | 22 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Euroasia Trans Continental | +91 22 56349035-36 | New Delhi, India | 518 | 47 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6905 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| QUALIKEMS FINE CHEM PVT LTD | 58 |
| Yogi Dye Chem Industries | 58 |
| Evans Fine Chem | 58 |
| Hemal Impex | 58 |
| Nithyasri Chemicals (APURVA CHEMICALS) | 58 |
| Eureka Chemicals | 58 |
| Cosmic Chemicals | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| Euroasia Trans Continental | 47 |
| Alfa Aesar | 58 |
3251-23-8(Copper dinitrate)Related Search:
1of4
chevron_right




