Silver acetate
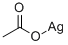
- CAS No.
- 563-63-3
- Chemical Name:
- Silver acetate
- Synonyms
- SILVER(I) ACETATE;ver acetate;Silver acetat;SILVER ACETATE;Acetoxysilver(I);Silveracetate,99%;silver(1+)acetate;silvermonoacetate;Silver acetate,90%;Ag64%Silver acetate
- CBNumber:
- CB1416235
- Molecular Formula:
- C2H3AgO2
- Molecular Weight:
- 166.91
- MOL File:
- 563-63-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | decomposes [STR93] |
|---|---|
| Density | 3.25 |
| storage temp. | Store below +30°C. |
| solubility | 10.2g/l |
| form | Powder/Solid |
| color | White to gray |
| Specific Gravity | 3.259 |
| Water Solubility | 10.2 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Hydrolytic Sensitivity | 4: no reaction with water under neutral conditions |
| Merck | 14,8505 |
| BRN | 3595636 |
| Solubility Product Constant (Ksp) | pKsp: 2.71 |
| Exposure limits |
ACGIH: TWA 0.01 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.01 mg/m3 |
| Stability | Stable, but light sensitive. Incompatible with strong reducing agents. |
| InChIKey | CQLFBEKRDQMJLZ-UHFFFAOYSA-M |
| CAS DataBase Reference | 563-63-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Silver acetate(563-63-3) |
| EPA Substance Registry System | Silver acetate (563-63-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335-H400 | |||||||||
| Precautionary statements | P261-P264-P271-P273-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,N | |||||||||
| Risk Statements | 36/37/38-50 | |||||||||
| Safety Statements | 26-36-37/39-61 | |||||||||
| RIDADR | 1759 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | AJ4100000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| HS Code | 28432900 | |||||||||
| NFPA 704 |
|
Silver acetate price More Price(18)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 921017 | Silver (I) Acetate anhydrous, ≥99.9% trace metals basis | 563-63-3 | 25G | ₹21844.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 921017 | Silver (I) Acetate anhydrous, ≥99.9% trace metals basis | 563-63-3 | 100G | ₹56143.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.01504 | Silver acetate for synthesis | 563-63-3 | 5G | ₹10960 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 85140 | Silver acetate purum p.a., ≥99.0% (T) | 563-63-3 | 10G | ₹8069.7 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.01504 | Silver acetate for synthesis | 563-63-3 | 25G | ₹15220 | 2022-06-14 | Buy |
Silver acetate Chemical Properties,Uses,Production
Description
Silver acetate is an organic compound with the empirical formula CH3COOAg (or AgC2H3O2). It is a photosensitive, white crystalline solid. It is a useful reagent in the laboratory as a water soluble source of silver lacking an oxidizing anion. It has been used in some antismoking drugs.
Chemical Properties
Off-White/Brown Crystalline Powder
Uses
In the health field, silver acetate-containing products have been used in gum, spray, and lozenges to deter smokers from smoking. The silver in these products, when mixed with smoke, creates an unpleasant metallic taste in the smoker's mouth, thus deterring them from smoking. Lozenges containing 2.5 mg of silver acetate showed "modest efficacy" on 500 adult smokers tested over a three-month period. However, over a period of 12 months, prevention failed. In 1974, silver acetate was first introduced in Europe as an over-thecounter smoking-deterrent lozenge (Repaton) and then three years later as a chewing gum (Tabmint).
Reactions
3 – 1 - Carbonylation
Silver acetate, when combined with carbon monoxide (CO), can induce the carbonylation of primary and secondary amines. Other silver salts can be used but the acetate gives the best yield.
2 R2NH + 2 AgOAc + CO → [R2N]2CO + 2 HOAc + 2 Ag
3 – 2 - Hydrogenation
Silver acetate in a solution of pyridine absorbs hydrogen and is reduced to metallic silver.
3 – 3 - Direct ortho - arylation
Silver acetate is a useful reagent for direct ortho-arylation (to install two adjacent substituents on an aromatic ring) for of benzylamines and N-methylbenzylamines. The reaction is palladiumcatalized and requires a slight excess of silver acetate.This reaction is shorter than previous ortho-arylation methods.
World Health Organization (WHO)
Silver acetate has been used as a disinfectant and as an antismoking aid. It was refused registration in Cyprus on the grounds that prolonged use of silver salts can cause permanent argyria and that no well-controlled trials have been performed to establish the safety and efficacy of the preparation. It remains registered as an aid to stopping smoking in Canada and the United States.
General Description
White crystalline plates. Light sensitive. Density 3.26 g / cm3.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Silver acetate is freely soluble in dilute nitric acid [Merck]. Can serve as an oxidizing agent.
Hazard
Toxic material.
Health Hazard
Inhalation of dust irritates nose and throat. Contact with eyes or skin causes irritation. If continued for a long period, ingestion or inhalation of silver compounds can cause permanent discoloration of skin (argyria).
Safety
The LD50 of silver acetate in mice is 36.7 mg/kg. Low doses of silver acetate in mice produced hyper-excitability, ataxia, central nervous system depression, labored breathing, and even death. The U.S. FDA recommends that silver acetate intake be limited to 756 mg over a short period of time; excessive intake may cause argyria.
Purification Methods
Shake it with acetic acid for three days, and the process is repeated with fresh acid. The solid is then dried in a vacuum oven at 40o for 48hours. It has also been recrystallised from water containing a trace of acetic acid, and dried in air. Store it in the dark. [Beilstein 2 IV 112.]
Silver acetate Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Evans Fine Chem | +91-9821340302 +91-9821340302 | Maharashtra, India | 286 | 58 | Inquiry |
| Dhara Industries | +91-9322395199 +91-9322395199 | Mumbai, India | 190 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| ARRAKIS INDUSTRIES LLP | +91 74995 32711 | Maharashtra, India | 1353 | 58 | Inquiry |
| Scientific OEM | +91-22- 2343 7546 / 2341 3094 | New Delhi, India | 1994 | 38 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6700 | 30 | Inquiry |
| FINAR LTD | +91 - 2717 - 616717 | New Delhi, India | 660 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5870 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4315 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Evans Fine Chem | 58 |
| Dhara Industries | 58 |
| JSK Chemicals | 58 |
| ARRAKIS INDUSTRIES LLP | 58 |
| Scientific OEM | 38 |
| CHEMSWORTH | 30 |
| FINAR LTD | 58 |
| A.J Chemicals | 58 |
| Otto Chemie Pvt. Ltd. | 58 |
| Sisco Research Laboratories Pvt. Ltd. | 58 |
563-63-3(Silver acetate )Related Search:
1of4
chevron_right




