Calcium acetate
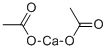
- CAS No.
- 62-54-4
- Chemical Name:
- Calcium acetate
- Synonyms
- teltozan;FEMA 2228;grayacetate;limeacetate;vinegarsalts;brownacetate;MAGGRAN(R) CA;sorbo-calcion;Calciumacetat;MAGNESIA 87219
- CBNumber:
- CB8312095
- Molecular Formula:
- C4H6CaO4
- Molecular Weight:
- 158.17
- MOL File:
- 62-54-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/29 16:45:47
| Melting point | 160°C (dec.) |
|---|---|
| Density | 1,5 g/cm3 |
| refractive index | 1.5500 |
| FEMA | 2228 | CALCIUM ACETATE |
| Flash point | 160°C |
| storage temp. | Hygroscopic, Room Temperature, under inert atmosphere |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Powder |
| color | white |
| Specific Gravity | 1.50 |
| PH | 8(1 mM solution);8.43(10 mM solution);8.77(100 mM solution);9.13(1000 mM solution) |
| Odor | mild acetic |
| Odor Type | acetic |
| Water Solubility | soluble |
| Decomposition | 160 ºC |
| Stability | Stable. Non-flammable. Incompatible with strong oxidizing agents. |
| InChIKey | VSGNNIFQASZAOI-UHFFFAOYSA-L |
| LogP | -0.29 |
| CAS DataBase Reference | 62-54-4(CAS DataBase Reference) |
| EPA Substance Registry System | Calcium acetate (62-54-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H335-H319 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | AF7525000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29152990 | |||||||||
| NFPA 704 |
|
Calcium acetate Chemical Properties,Uses,Production
Description
Calcium acetate is a chemical compound which is calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous form is very hygroscopic; therefore the monohydrate (Ca(CH3COO)2?H2O) is the common form.
Chemical Properties
Calcium acetate occurs as a white or almost white, odorless or almost odorless, hygroscopic powder.
Uses
Calcium Acetate is the calcium salt of acetic acid which functions as a sequestrant and mold control agent. it contains approximately 25% calcium. it is a white odorless powder which is readily soluble in water with a solubility of approximately 37 g in 100 g water at 0°c. its solubility decreases with increasing temperature, with a sol- ubility of approximately 29 g in 100 g of water at 100°c.
Preparation
Produced by calcium hydroxide neutralization of acetic acid.
Production Methods
Calcium acetate can be prepared by soaking calcium carbonate (found in eggshells, or in common carbonate rocks such as lime stone or marble) in vinegar:
CaCO3 + 2CH3COOH → Ca(CH3COO)2 + H2O + CO2
Since both reagents would have been available pre-historically, the chemical would have been observable as crystals then.
Definition
ChEBI: The calcium salt of acetic acid. It is used, commonly as a hydrate, to treat hyperphosphataemia (excess phosphate in the blood) in patients with kidney disease: the calcium ion combines with dietary phosphate to form (insoluble) calcium phosphate, which is excreted in the faeces.
General Description
Calcium Acetate belongs to the group of calcium salts, widely used as phosphorus binders in patients with chronic renal failure.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical Applications
Calcium acetate is used as a preservative in oral and topical
formulations.
Therapeutically, parenteral calcium acetate acts as a source of
calcium ions for hypocalcemia or electrolyte balance. Oral
calcium acetate is used as a complexing agent for hyperphosphatemia
in dialysis patients. Calcium acetate is also used in the
food industry as a stabilizer, buffer and sequestrant.
Safety Profile
Poison by intravenous and intraperitoneal routes. Mutation data reported. See also CALCIUM COMPOUNDS. When heated to decomposition it emits acrid smoke and fumes.
Safety
Calcium acetate is used in oral and topical formulations. The pure
form of calcium acetate is toxic by IP and IV routes.
LD50 (mouse, IP): 0.075 g/kg
LD50 (mouse, IV): 0.052 g/kg
LD50 (rat, oral): 4.28 g/kg
storage
Calcium acetate is stable although very hygroscopic, and so the
monohydrate is the common form. It decomposes on heating (above
1608℃) to form calcium carbonate and acetone.
Store in well-closed airtight containers.
Purification Methods
Recrystallise it from water (3mL/g) by partial evaporation in a desiccator. [Beilstein 2 IV 113.]
Incompatibilities
Calcium acetate is incompatible with strong oxidizing agents and moisture.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral suspensions and tablets; topical emulsions, lotions, and creams). Included in nonparenteral medicines (oral tablets) licensed in the UK.
Calcium acetate Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SAGAR LIFE SCIENCES PVT LTD | +91-8770359041 +91-8770359041 | Gujarat, India | 79 | 58 | Inquiry |
| Indiana ChemPort | +91-91-2652638667 +91-7574886711 | Gujarat, India | 85 | 58 | Inquiry |
| QUALIKEMS FINE CHEM PVT LTD | +911123618475 | New Delhi, India | 51 | 58 | Inquiry |
| S.V ENTERPRISES | +919322701159 | Mumbai, India | 150 | 58 | Inquiry |
| PERFECT CHEMICAL | +91-9820465461 +91-9820465461 | Mumbai, India | 391 | 58 | Inquiry |
| Mahadev Pharmaceuticals | +912646272307 | Gujarat, India | 26 | 58 | Inquiry |
| Evans Fine Chem | +91-9821340302 +91-9821340302 | Maharashtra, India | 286 | 58 | Inquiry |
| SAANVI CORP | +91-7208939370 +91-7208939370 | Maharashtra, India | 46 | 58 | Inquiry |
| AVA CHEMICALS PVT LTD | +91-8879390046 +91-8879390046 | Maharashtra, India | 74 | 58 | Inquiry |
| Kronox Lab Sciences Pvt Ltd | +91-9313231074 +91-9313231074 | Gujarat, India | 240 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| SAGAR LIFE SCIENCES PVT LTD | 58 |
| Indiana ChemPort | 58 |
| QUALIKEMS FINE CHEM PVT LTD | 58 |
| S.V ENTERPRISES | 58 |
| PERFECT CHEMICAL | 58 |
| Mahadev Pharmaceuticals | 58 |
| Evans Fine Chem | 58 |
| SAANVI CORP | 58 |
| AVA CHEMICALS PVT LTD | 58 |
| Kronox Lab Sciences Pvt Ltd | 58 |





