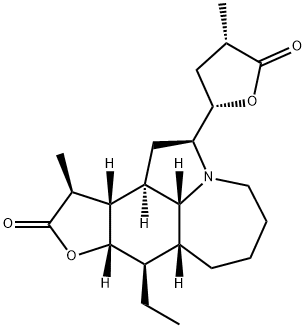
tuberostemonine
- CAS No.
- 6879-01-2
- Chemical Name:
- tuberostemonine
- Synonyms
- Nsc366235;Parathymine;uberostemonine;TuberosteMonin;tuberostemonine;TUBERSTEMONINE(P);Tuberostemonine, 98%, from Stemona tuberosa L.;2β-[(2S,4S)-Tetrahydro-4-methyl-5-oxofuran-2-yl]stenine;NADP+,Parasite,Tuberostemonine,Inhibitor,antimalarial,pfFNR,reductases,falciparum,inhibit,Plasmodium,ferredoxin;(2S,31R,7AR,8R,8aS,11S,11aS,11bR)-8-Ethyl-11-methyl-2-((2S,4S)-4-methyl-5-oxotetrahydrofuran-2-yl)dodecahydroazepino[3,2,1-hi]furo[3,2-e]indol-10(2H)-one
- CBNumber:
- CB21372283
- Molecular Formula:
- C22H33NO4
- Molecular Weight:
- 375.5
- MOL File:
- 6879-01-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:02
| Melting point | 86~88℃ |
|---|---|
| Boiling point | 554.2±50.0 °C(Predicted) |
| Density | 1.19±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C(protect from light) |
| solubility |
DMF:10.0(Max Conc. mg/mL);26.63(Max Conc. mM) DMF:PBS (pH 7.2) (1:2):0.33(Max Conc. mg/mL);0.88(Max Conc. mM) DMSO:1.0(Max Conc. mg/mL);2.66(Max Conc. mM) |
| form | A solid |
| pka | 8.90±0.70(Predicted) |
| color | White to off-white |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P321-P362+P364-P332+P313-P337+P313-P403+P233-P405-P501 | |||||||||
| HS Code | 29399990 | |||||||||
| NFPA 704 |
|
tuberostemonine Chemical Properties,Uses,Production
Description
This novel alkaloid has been isolated from Sternona sessilifolia and S. tuberosa and was first given the formula C19H29O4N, subsequently altered to that given above. It crystallizes in colourless needles from MeOH with 1 mole of solvent, m.p. 65-88°C (dec.) and has [α]D - 25.4° (Me2CO). It is a non-phenolic, tertiary base and yields a hydrobromide, m.p. 120°C (dec.) and a perchlorate, m.p. 242°C (dec.). The methiodide monohydrate has m.p. 236-8°C (dec.) and the methochloride, dihydrate, m.p. 172°C. A methosulphate has also been prepared, m.p. 253°C (dec.). The base is not hydrolyzed by acids but in the presence of excess PtO2 it gives the dihydro derivative, m.p. 133°C, furnishing a hydrochloride, m.p. 281 ° C. Oxidation with KMnO4 gives a C17 compound, whereas oxidation with silver oxide forms a neutral substance, C22H29O4N, m.p. 178°C which still contains the lactone groups and gives a positive Ehrlich pyrrole reaction.
Uses
Tuberostemonine is elucidated as one of the major components of Radix Stemonae and has been shown to display therapeutic effects against cigarette smoke-induced acute lung inflammation.
Definition
ChEBI: A natural product found in Stemona phyllantha and Stemona tuberosa.
References
Kondo, Suzuki, Satomi.,J. Pharrn. Soc., Japan, 54,96 (1934)
Kondo, Suzuki, Satomi., ibid, 59, 177 (1939)
Kondo, Suzuki, Satomi., ibid, 60, 149 (1940)
Kondo, Suzuki, Satomi., ibid, 61, 111 (1941)
Kaneko., Ann. Rep. ITSUU Lab. (Tokyo), 11,45 (1960)
Gotz, Bogri, Gray., Tetrahedron Lett., 707 (1961)
Edwards, Feniak, Handa., Can. J. Chern., 40,455,2416 (1962)
Crystal structure:
Harada et ai., Chern. Cornrnun., 460 (1967)
tuberostemonine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | China | 1000 | 58 | Inquiry |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | CHINA | 1824 | 58 | Inquiry |
| Chengdu Biopurify Phytochemicals Ltd. | +86-28-82633860 +86-18080483897 | China | 3424 | 58 | Inquiry |
| Shaanxi Pioneer Biotech Co., Ltd . | +8613259417953 | China | 3000 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | China | 11667 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |





