Fluorosilicate
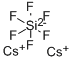
- CAS No.
- 17084-08-1
- Chemical Name:
- Fluorosilicate
- Synonyms
- fluosilicate;silicofluoride;fluorosilicate;hexafluorosilicate;16893-85-9 (Sodium);17125-80-3 (Barium);16925-39-6 (Calcium);16919-19-0 (Ammonium);16949-65-8 (Magnesium);16871-90-2 (Potassium)
- CBNumber:
- CB2258629
- Molecular Formula:
- Cs2F6Si
- Molecular Weight:
- 407.89
- MOL File:
- 17084-08-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/12/18 14:07:02
| Density | 4.75 g/cm3 |
|---|---|
| Solubility Product Constant (Ksp) | pKsp: 4.9 |
| EPA Substance Registry System | Silicate(2-), hexafluoro- (17084-08-1) |
Fluorosilicate Chemical Properties,Uses,Production
Chemical Properties
More soluble than the corresponding fluoride salt.
Definition
Any salt of fluorosilic acid.
General Description
A crystalline solid or the solid dissolved in a liquid. Denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals.
Air & Water Reactions
Slightly soluble in water. Forms heavy clouds with moist air, decomposed by water into silicic acid and hydrofluoric acid [Merck 11th ed. 1989].
Reactivity Profile
Salts, basic, such as SILICOFLUORIDES, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Hazard
Very toxic.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Fluorosilicate Preparation Products And Raw materials
Raw materials
Preparation Products
Fluorosilicate Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | China | 34563 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 7859 | 58 | Inquiry |
| XIAMEN AMITY INDUSTRY AND TRADE CO., LTD. | +8618950047208 | China | 43416 | 58 | Inquiry |
| DAYANG CHEM (HANGZHOU) CO.,LTD | +8617705817739 | China | 53881 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39927 | 58 | Inquiry |
| Aobolihui | 0917-18691729 18691729963 | China | 4746 | 58 | Inquiry |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 029-81120477 17792793610 | China | 9763 | 58 | Inquiry |
| Pushan Industry (Shaanxi) Co., Ltd. | 029-81310890 13571859809 | China | 10004 | 58 | Inquiry |
| Hubei Baidu Chemical Co., Ltd | 027-59106051 13627137652 | China | 9616 | 58 | Inquiry |
| Hubei Chenghai Chemical Co., Ltd | 18327245847 18327245847 | China | 9918 | 58 | Inquiry |
17084-08-1(Fluorosilicate)Related Search:
1of2
chevron_right




