Triphosgene
- CAS No.
- 32315-10-9
- Chemical Name:
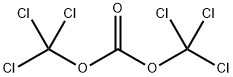
- Triphosgene
- Synonyms
- BIS(TRICHLOROMETHYL) CARBONATE;TRIPHOSGEN;Triphosg;Triphosgene, BTC;BIS(TRICHLOROMETHYL) CARBONATE FOR SYNTH;Triphosgene, 98% [Bis-(trichloromethyl)-carbonat];TRIPHOSGENE;triphosqene;Triphosgene98+%;Triphosgene,99%
- CBNumber:
- CB2420273
- Molecular Formula:
- C3Cl6O3
- Molecular Weight:
- 296.73
- MOL File:
- 32315-10-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/21 22:41:43
| Melting point | 79-83 °C (lit.) |
|---|---|
| Boiling point | 203-206 °C (lit.) |
| Density | 1.78 |
| vapor pressure | 16 hPa (90 °C) |
| Flash point | 203-206°C |
| storage temp. | 2-8°C |
| solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. |
| form | Crystalline Powder, Crystals and/or Chunks |
| color | White to off-white |
| Water Solubility | practically insoluble |
| Sensitive | Moisture Sensitive |
| BRN | 1787583 |
| InChIKey | UCPYLLCMEDAXFR-UHFFFAOYSA-N |
| CAS DataBase Reference | 32315-10-9(CAS DataBase Reference) |
| EPA Substance Registry System | Methanol, trichloro-, carbonate (2:1) (32315-10-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314-H330 | |||||||||
| Precautionary statements | P260-P271-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T+,Xn,T | |||||||||
| Risk Statements | 26-34-29-36/37/38-20/21/22-23/24/25 | |||||||||
| Safety Statements | 36/37/39-45-9-26-36-7/9 | |||||||||
| RIDADR | UN 2928 6.1/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| F | 10-19-21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29209010 | |||||||||
| NFPA 704 |
|
Triphosgene price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 8.14283 | Bis(trichloromethyl) carbonate for synthesis | 32315-10-9 | 5G | ₹2990 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.14283 | Bis(trichloromethyl) carbonate for synthesis | 32315-10-9 | 25G | ₹6880 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.14283 | Bis(trichloromethyl) carbonate for synthesis | 32315-10-9 | 100G | ₹8650 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.14283 | Bis(trichloromethyl) carbonate for synthesis | 32315-10-9 | 1KG | ₹15290 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 330752 | Triphosgene reagent grade, 98% | 32315-10-9 | 5G | ₹3344.93 | 2022-06-14 | Buy |
Triphosgene Chemical Properties,Uses,Production
Description
Triphosgene is also known as solid phosgene. Its chemical name is bis (Trichloromethyl) carbonate, and its English name is bisgriehloromethyl) carbonate or triphosgene, abbreviated as BTC. Triphosgene is a white crystal, similar to the smell of phosgene. It is mainly used to synthesize chloroformate, isocyanate, polycarbonate and acyl chloride. It is widely used as an intermediate in plastics, medicine, herbicides and pesticides.
Chemical Properties
White solid
Uses
Triphosgene is used as a carbonylating agent for aza-peptide synthesis. It reacts with several alfa-amino acids to give the corresponding N-carboxyanhydrides. It is involved in the preparation of the esterification coupling reagent, di-2-thienyl carbonate from 2(5H)-thiophenone. Further, it is used as a reagent in organic synthesis and converts an amino group into isocyanate. In addition to this, it is employed in the preparation of 2-chloronicotinaldehydes through cyclization of the corresponding enamides. It is considered as a useful substitute for phosgene.
Reactions
The Triphosgene reaction is quenched very carefully by dropwise addition of 75 mL of saturated aqueous sodium sulfate solution (Notes 5 and 6). The resulting white granular precipitate or slurry is removed by vacuum filtration through Celite, and the filter cake is washed with three portions of 75 mL of chloroform .
General Description
Triphosgene [Bis(trichloromethyl)carbonate] is a versatile organic reagent used in organic synthesis alternative to phosgene. A catalytic amount of triphosgene is particularly used in chloroformylations, carbonylations, chlorinations, and dehydration reactions.
Safety
Triphosgene's low vapor pressure makes it possible for it to reach concentrations that are considered toxicologically unsafe. While several properties of triphosgene are not yet readily available, it is known that it is extremely toxic if inhaled. A toxic gas is emitted if it comes in contact with water. There is a lack of information and variability regarding the proper handling of triphosgene. It is assumed to have the same risks as phosgene.
Synthesis
Triphosgene is synthesized by exhaustive free radical chlorination of dimethyl carbonate:
CH3OCO2CH3 + 6 Cl2 → CCl3OCO2CCl3 + 6 HCl
Triphosgene can be easily recrystallized from hot hexanes.
Purification Methods
It is a good solid substitute for phosgene (using a third mol per mol). Crystallise it from pet ether (b 60-80o), wash it with anhydrous cold Et2O, de-gas it at 200mm then dry at 0.1mm (over H2SO4). It has IR: max 900 and 1900 cm-1 . It is a lachrymator, is TOXIC and should be handled with gloves and in an efficient fume hood. [Hales et al. J Chem Soc 620 1957, Eckert & Forster Angew Chem, Int Ed Engl 26 894 1987, Aldrichimica Acta 21 47 1988, Beilstein 3 H 17, 3 I 8, 3 II 16, 3 III 36, 3 IV 33.]
Triphosgene Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ULTIMA CHEMICALS | +91 7208029458 | Mumbai, India | 95 | 58 | Inquiry |
| SUJALAM CHEMICALS | +91-9422560788 +91-9422560788 | Maharashtra, India | 42 | 58 | Inquiry |
| Evans Fine Chem | +91-9821340302 +91-9821340302 | Maharashtra, India | 286 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Otto Chemie Pvt Ltd | +91-2222070099 +91-2266382599 | Maharashtra, India | 429 | 58 | Inquiry |
| Dhara Industries | +91-9322395199 +91-9322395199 | Mumbai, India | 190 | 58 | Inquiry |
| ADAARSH PHARMA | +91-9133632777 +91-9133632777 | Karnataka, India | 88 | 58 | Inquiry |
| A. B. Enterprises | 08048986961 | Mumbai, India | 226 | 58 | Inquiry |
| Shri Raj Industries | 91-79-65052777 | Gujarat, India | 47 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ULTIMA CHEMICALS | 58 |
| SUJALAM CHEMICALS | 58 |
| Evans Fine Chem | 58 |
| JSK Chemicals | 58 |
| Otto Chemie Pvt Ltd | 58 |
| Dhara Industries | 58 |
| ADAARSH PHARMA | 58 |
| A. B. Enterprises | 58 |
| Shri Raj Industries | 58 |
| SynZeal Research Pvt Ltd | 58 |
Related articles
- Triphosgene: History and Advantage
- Triphosgene, or bis(trichloromethyl) carbonate or BTC, is a convenient substitute for the extremely toxic phosgene gas.
- Aug 9,2024
- Introduction of Triphosgene
- Triphosgene is also known as solid phosgene. Its chemical name is bis (Trichloromethyl) carbonate, and its English name is bis....
- Jan 25,2022
32315-10-9(Triphosgene)Related Search:
1of4
chevron_right




