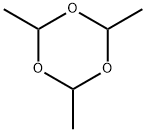
Paraldehyde
- CAS No.
- 123-63-7
- Chemical Name:
- Paraldehyde
- Synonyms
- ALDEHYDE;2,4,6-TRIMETHYL-1,3,5-TRIOXANE;ETHYL ALDEHYDE;ACETIC ALDEHYDE;Paral;FEMA 2003;Pcho;NSC 9799;ALDEFRESH;paradehyde
- CBNumber:
- CB2716567
- Molecular Formula:
- C6H12O3
- Molecular Weight:
- 132.16
- MOL File:
- 123-63-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/11/28 16:31:44
| Melting point | 12 °C |
|---|---|
| Boiling point | 65-82 °C |
| Density | 0.994 g/mL at 20 °C (lit.) |
| vapor density | 1.52 (vs air) |
| vapor pressure | 25.89 psi ( 55 °C) |
| FEMA | 4010 | PARALDEHYDE |
| refractive index |
n |
| Flash point | 30 °F |
| storage temp. | 2-8°C |
| solubility | 120g/l |
| pka | 16(at 25℃) |
| form | solution |
| Specific Gravity | 0.994 |
| color | Colorless liquid |
| Odor | disagreeable taste, aromatic odor |
| explosive limit | 1.3-17.0%(V) |
| Water Solubility | 125 g/L (25 ºC) |
| Merck | 13,7098 |
| BRN | 80142 |
| Dielectric constant | 14.5(20℃) |
| Stability | Stable. Flammable. Incompatible with strong oxidizing agents, mineral acids. |
| InChIKey | SQYNKIJPMDEDEG-UHFFFAOYSA-N |
| LogP | 0.670 |
| CAS DataBase Reference | 123-63-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Paraldehyde(123-63-7) |
| EPA Substance Registry System | Paraldehyde (123-63-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P242-P243 | |||||||||
| Hazard Codes | F | |||||||||
| Risk Statements | 11-R11-10 | |||||||||
| Safety Statements | 9-16-29-33-S9-S33-S29-S16 | |||||||||
| RIDADR | UN 1993 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | YK0525000 | |||||||||
| Autoignition Temperature | 235 °C | |||||||||
| HazardClass | 3.2 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29125000 | |||||||||
| Toxicity | LD50 orally in rats: 1.65 g/kg (Figot) | |||||||||
| NFPA 704 |
|
Paraldehyde price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TCI Chemicals (India) | P0019 | Paraldehyde min. 98.0 % | 123-63-7 | 25ML | ₹2200 | 2022-05-26 | Buy |
| TCI Chemicals (India) | P0019 | Paraldehyde min. 98.0 % | 123-63-7 | 500ML | ₹3400 | 2022-05-26 | Buy |
| ottokemi | P 1338 | Paraldehyde 97% | 123-63-7 | 500mL | ₹1584 | 2022-05-26 | Buy |
Paraldehyde Chemical Properties,Uses,Production
Description
Paraldehyde was first synthesized in 1829 by Wildenbusch and introduced into clinical practice in the United Kingdom by the Italian physician Vincenzo Cervello in 1882. Paraldehyde, a polymer of acetaldehyde, is a clear colorless or slightly yellow transparent liquid with a strong aromatic as well as a disagreeable taste that at low temperatures, it solidifies into a crystalline mass. This agent decomposes with strong into toxic products that may be released into water, soil, or atmosphere.
Chemical Properties
Paraldehyde is a colourless to yellowish clear liquid. Pleasant, sweet odor. Less dense than water. Vapors are heavier than air.
Uses
Paraldehyde is used as solvent for fats, oils, waxes, rubber and resins; as substitute for acetaldehyde; as intermediate for organic chemicals, dyestuffs, accelerators for vulcanizations, rubber oxidants, etc. Product Data Sheet
Preparation
Prepared by the polymerization of acetaldehyde catalyzed by hydrochloric acid and sulfuric acid at medium to high temperatures.
Definition
ChEBI: Paraldehyde is a trioxane that is 1,3,5-trioxane substituted by methyl groups at positions 2, 4 and 6. It has a role as a sedative.
General Description
Paraldehyde is recognizable as the cyclictrimer of acetaldehyde. It is a liquid with a strong characteristicodor detectable in the expired air and an unpleasanttaste. These properties limit its use almost exclusively toan institutional setting (e.g., in the treatment of deliriumtremens). In the past, when containers were opened and airadmitted and then reclosed and allowed to stand, fatalitiesoccurred because of oxidation of paraldehyde to glacialacetic acid.
Air & Water Reactions
Highly flammable. Slightly soluble in water. Decomposed by light and air, on prolonged storage, to acetaldehyde and acetic acid.
Reactivity Profile
Paraldehyde is an aldehyde. Paraldehyde is decomposed by light and air, on prolonged storage, to acetaldehyde and acetic acid. Incompatible with alkalis, hydrocyanic acid iodides and oxidizers.
Health Hazard
INHALATION AND INGESTION: Irritation, headache, bronchitis, pulmonary edema. Irritating to digestive tract. Hypnotic and analgesic properties. Incoordination and drowsiness, followed by sleep. Larger doses-coma-weak pulse and shallow respiration, cyanosis-death from respiratory paralysis. EYES: irritation-can cause serious injury. SKIN: Dermatitis (skin inflammation).
Safety Profile
A human poison by rectal route. Moderately toxic to humans by intramuscular route. Moderately toxic experimentally by inhalation, ingestion, intraperitoneal, and subcutaneous routes. Human systemic effects by rectal route: necrotic changes. A skin and severe eye irritant. Low doses produce hypnotic and analgesic effects. Larger doses depress the nervous system with loss of reflexes, coma, and respiratory depression leadmg to respiratory paralysis and death. Chronic effects include weight loss, muscular weakness, and mental fatigue. However, poisoning is rare. A hypnotic agent. Dangerous fire hazard when exposed to heat, flame, or oxidzers. Slight explosion hazard when exposed to heat or flame. Dangerous; keep away from heat and open flame. To fight fire, use alcohol foam, CO2, dry chemical. Potentially violent reaction with nitric acid. Incompatible with alkahes, hydrocyanic acid, iodides, oxidzers. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALDEHYDES
Potential Exposure
Paraldehyde is used primarily in medicine. It is used as a hypnotic agent, in delirium tremens; and in treatment of psychiatric states characterized by excitement when drugs must be given over a long period of time. It also is administered for intractable pain which does not respond to opiates and for basal and obstetrical anesthesia. It is effective against experimentally induced convulsions and has been used in emergency therapy of tetanus, eclampsia, status epilepticus; and poisoning by convulsant drugs. Since it is used primarily in medicine, the chance of accidental human exposure or environmental contamination is low. However, paraldehyde decomposes to acetaldehyde and acetic acid; these compounds have been found to be toxic. In this case, occupational exposure or environmental contamination is possible. Since paraldehyde is prepared from acetaldehyde by polymerization in the presence of an acid catalyst, there exists a potential for adverse effects, although none have been reported in the available literature. It is also used in the manufacture of organic compounds.
Environmental Fate
Paraldehyde is used in resin manufacture, as a preservative, and in other processes as a solvent. Paraldehyde may enter to the environment via industrial effluents or hospital wastes. Acetaldehyde and acetic acid are two products of degradation of paraldehyde. This compound and its degradation products may be released into water, soil, or atmosphere and then they may be removed from the atmosphere by precipitation.
Shipping
UN1264 Paraldehyde, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
Wash paraldehyde with water and fractionally distil it. Alternatively, it is purified by drying with anhydrous Na2SO4, then cooled to 5o, and the frozen material is separated by decantation. The solid is distilled (b 121-124o/atm), the distillate is collected, stored over anhydrous Na2SO4 for several days and re-distilled at atmospheric pressure before use [Le Fevre et al. J Chem Soc 290 1950]. The 2r,4c,6t-trimethyl-1,3,5-trioxane has m 14.5o, b 125o/760mm. [Beilstein 19 II 394, 19 III/IV 4715. 19/9 V 112.]
Incompatibilities
Vapor or liquid may form explosive mixture with air. Incompatible with strong oxidants, strong acids; alkalis, ammonia, amines, iodides, hydrocyanic acid. Violent reaction with liquid oxygen. Contact with acids form acetaldehyde. Attacks rubber and plastics.
Waste Disposal
Incineration in added solvent. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Paraldehyde Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GODAVARI BIOREFINERIES LTD | 9769972334 | Maharashtra, India | 26 | 58 | Inquiry |
| Ashok Chem-Pharma International | 91-22-40627200 | Maharashtra, India | 12 | 58 | Inquiry |
| Godavari Biorefineries Limited | 08066085808Ext 330 | Mumbai, India | 15 | 58 | Inquiry |
| Oceanic Pharmachem Pvt., Ltd. (OPPL) | 91-22-42128600 | Maharashtra, India | 947 | 58 | Inquiry |
| HiMedia Laboratories | 91-22-61471919 | Maharashtra, India | 1843 | 58 | Inquiry |
| Loba Chemie Pvt., Ltd. | 91-22-66636663 | Maharashtra, India | 766 | 58 | Inquiry |
| Oxford laboratories | +91 250 239 0032 | Maharashtra, India | 652 | 58 | Inquiry |
| Innovative | 07942565925 | Mumbai, India | 617 | 58 | Inquiry |
| Loba Chemie Pvt Ltd | +91-22-6663 6663 | Mumbai, India | 498 | 58 | Inquiry |
| Godavari Biorefineries Ltd - GBL (Somaiya Group) | 91-22-61702100 | Maharashtra, India | 18 | 58 | Inquiry |
Related articles
- Paraldehyde - Uses, Side Effects, and Environmental Fate
- Paraldehyde was first synthesized in 1829 by Wildenbusch and introduced into clinical practice in the United Kingdom by the It....
- Nov 4,2021
123-63-7(Paraldehyde)Related Search:
1of4
chevron_right




