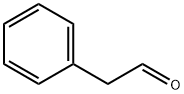
Phenylacetaldehyde
- CAS No.
- 122-78-1
- Chemical Name:
- Phenylacetaldehyde
- Synonyms
- Benzeneacetaldehyde;2-PHENYLACETALDEHYDE;Hyacinthin;Phenylethanal;2-Phenylethanal;phenylacetadehyde;FEMA 2874;α-Tolualdehyde;ALPHA-TOLUALDEHYDE;Benzenacetaldehyde
- CBNumber:
- CB7852954
- Molecular Formula:
- C8H8O
- Molecular Weight:
- 120.15
- MOL File:
- 122-78-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/24 19:19:26
| Melting point | −10 °C(lit.) |
|---|---|
| Boiling point | 195 °C |
| Density | 1.079 g/mL at 20 °C |
| vapor pressure | 2.09hPa at 20℃ |
| refractive index |
n |
| FEMA | 2874 | PHENYLACETALDEHYDE |
| Flash point | 188 °F |
| storage temp. | 2-8°C |
| solubility | 2.21g/l slightly soluble |
| form | Liquid |
| color | Clear colorless to pale yellow |
| Specific Gravity | 1.075 (20/4℃) |
| Odor | at 10.00 % in dipropylene glycol. green sweet floral hyacinth clover honey cocoa |
| Odor Type | green |
| Water Solubility | 2.210 g/L (25 ºC) |
| Sensitive | Air Sensitive |
| JECFA Number | 1002 |
| Merck | 14,7265 |
| BRN | 385791 |
| Dielectric constant | 4.8(20℃) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases. |
| LogP | 1.44 at 25℃ |
| CAS DataBase Reference | 122-78-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzeneacetaldehyde(122-78-1) |
| EPA Substance Registry System | Phenylacetaldehyde (122-78-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H314-H317-H412 | |||||||||
| Precautionary statements | P261-P273-P280-P301+P312-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,F | |||||||||
| Risk Statements | 22-36/37/38-43-11 | |||||||||
| Safety Statements | 26-36-37-24-16-7 | |||||||||
| RIDADR | UN 1170 3/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | CY1420000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29122990 | |||||||||
| Toxicity | LD50 orl-rat: 1550 mg/kg FCTXAV 17,377,79 | |||||||||
| NFPA 704 |
|
Phenylacetaldehyde price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | W287407 | Phenylacetaldehyde ≥95%, FCC, FG | 122-78-1 | 1SAMPLE-K | ₹5141.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W287407 | Phenylacetaldehyde ≥95%, FCC, FG | 122-78-1 | 1KG | ₹17872.08 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W287407 | Phenylacetaldehyde ≥95%, FCC, FG | 122-78-1 | 5KG | ₹67017.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W287407 | Phenylacetaldehyde ≥95%, FCC, FG | 122-78-1 | 10KG | ₹104060.73 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.43654 | Phenyl acetic aldehyde for synthesis | 122-78-1 | 100ML | ₹7270.01 | 2022-06-14 | Buy |
Phenylacetaldehyde Chemical Properties,Uses,Production
Description
Phenylacetaldehyde is an organic compound which can be naturally found in buckwheat, flowers, and communication pheromones from various insect orders. It is commonly used for the preparation of fragrance as well as flavors, and applied in flavored cigarettes and beverages because of its honey-like sweet character. It is also applied in the synthesis of polymers, such as polyesters, as a rate controlling additive in polymerization reactions and used in the preparation of more complex chemicals like resmethrin, where it acts as a building block.
Chemical Properties
Phenylacetaldehyde has a harsh, green odor reminiscent of hyacinth on dilution. It has an unpleasant, pungent, bitter flavor, turning sweet and fruit-like at low levels. Since it readily undergoes oxidation and polymerizes, it must be stabilized by addition of antioxidants and by dilution with, for example, diethyl phthalate before use in compositions.
Occurrence
Identified among the constituents of several essential oils: neroli, Citrus sinensis leaves, other citrus species (flowers and leaves), narcissus, magnolia, lily, rose and tea. It is reported found in over 170 natural products including apricot, sour cherry, cooked apple, peach, fresh blackberry, crispbread, other types of bread, green tea, unprocessed rice, lemon balm, red sage, black currant, bilberry, cranberry, other berries, grapes, raisins, melon, papaya, guava fruit, pineapple, asparagus, celery leaves, carrot, parsley, peas, bell pepper, sweet pepper, peach, cabbage, peppermint oil, Scotch spearmint oil, mustard, vinegar, onion, cooked potato, tomato, cinnamon bark, cassia leaf, ginger, many cheeses, milk, yogurt, boiled egg, cooked and cured meats, beer, cognac, grape wines, cocoa, coffee, tea, roasted filbert, roasted peanut, soybean, pecans, cauliflower, broccoli, honey, avocado, passion fruit, beans, mushrooms, trassi, mango, tamarind, rice, licorice, buckwheat, lovage root, pumpkin, sweet potato, cassava, corn oil, malt, wort, dried bonito, loquat, pawpaw, maté, sweet grass oil, orange peel oil, grapefruit juice, endive, clam and Chinese quince.
Uses
Phenylacetaldehyde is used for the preparation of fragrances and polymers like polyesters, which find application as a rate controlling additive in polymerization reactions. It is an active component of fragrances and floral scent due to its honey-like sweet character and finds application in flavored cigarettes and beverages. It is an insect attractant and utilized in blacklight trap for pests. It is also used as a building block in the synthesis of more complex chemicals, such as resmethrin. Furthermore, it is used in association with acetic anhydride and allyltrimethylsilane in three-component coupling process catalyzed by LiBF4 providing homoallylic acetates.
Definition
ChEBI: Phenylacetaldehyde is an aldehyde that consists of acetaldehyde bearing a methyl substituent; the parent member of the phenylacetaldehyde class of compounds. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is an alpha-CH2-containing aldehyde and a member of phenylacetaldehydes.
General Description
Phenylacetaldehyde is an important aroma volatile found in tomato and roses. It has also been identified in potato, roasted cocoa beans and honey. Phenylacetaldehyde is also a potent moth attractant.
Safety Profile
Moderately toxic by ingestion. Human skin irritant. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALDEHYDES.
Synthesis
Phenylacetaldehyde can be synthesized by Darzen glycidic ester synthesis from benzaldehyde; readily oxidizable to phenyl acetic acid.
Production Methods
The most important method industrially is the isomerization of styrene oxide. Ion-exchange resins, catalysis by chromium trioxide–tungsten trioxide on graphite or silicon dioxide–aluminum trioxide, and thermolysis are recommended for the rearrangement. Phenylacetaldehyde can also be synthesized by the direct oxidation of styrene in the presence of palladium salts and copper(II) chloride in aqueous solutions of glycol ethers. Other possibilities include the catalytic dehydrogenation of 2-phenylethanol on silver or gold catalysts, the hydroformylation of benzyl halides in the presence of dicobalt octacarbonyl and sodium carbonate in acetonitrile, and the Darzens glycide ester synthesis from benzaldehyde and alkyl chloroacetates.
Phenylacetaldehyde Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Flowersynth | +91-9845115120 +91-9886340888 | Karnataka, India | 45 | 58 | Inquiry |
| Rossari Biotech Ltd. | +91-2261233800 +91-2261233800 | Mumbai, India | 57 | 58 | Inquiry |
| Aamirav Ingredients And Specialties Pvt Ltd | +91-2224980102 +91-9820077171 | Maharashtra, India | 138 | 58 | Inquiry |
| HRV Global Life Sciences | +91-9820219686 +91-9820219686 | Telangana, India | 379 | 58 | Inquiry |
| Insta Chemi Private Limited | 08048958481 | Uttar Pradesh, India | 122 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Dr Prem's Molecules Pvt. Ltd. | +91 9825310038 | New Delhi, India | 547 | 50 | Inquiry |
| Biokemix | +91 (40) 6662-6366 | New Delhi, India | 487 | 50 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Flowersynth | 58 |
| Rossari Biotech Ltd. | 58 |
| Aamirav Ingredients And Specialties Pvt Ltd | 58 |
| HRV Global Life Sciences | 58 |
| Insta Chemi Private Limited | 58 |
| A.J Chemicals | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| Dr Prem's Molecules Pvt. Ltd. | 50 |
| Biokemix | 50 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
122-78-1(Phenylacetaldehyde)Related Search:
1of4
chevron_right




