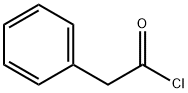
Phenylacetyl chloride
- CAS No.
- 103-80-0
- Chemical Name:
- Phenylacetyl chloride
- Synonyms
- phenylacetyl;Benzeneacetyl chloride;-Toluylchloride;A-TOLUYL CHLORIDE;AKOS BBS-00003909;ylacetyl chloride;PHENACETYL CHLORIDE;Phenyl acetyl chlori;henylacetyl chloride;Ethephon Impurity 14
- CBNumber:
- CB7852631
- Molecular Formula:
- C8H7ClO
- Molecular Weight:
- 154.59
- MOL File:
- 103-80-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/9/11 18:40:39
| Melting point | 264-266 °C(Solv: N,N-dimethylformamide (68-12-2)) |
|---|---|
| Boiling point | 94-95 °C/12 mmHg (lit.) |
| Density | 1.169 g/mL at 25 °C (lit.) |
| refractive index |
n |
| Flash point | 217 °F |
| storage temp. | 2-8°C |
| solubility | Miscible with alcohol and ether. |
| form | clear liquid |
| color | Colorless to Light yellow |
| Sensitive | Moisture Sensitive |
| BRN | 742254 |
| Stability | Stable. Reacts with water. Incompatible with amines, most common metals, moisture, strong oxidizing agents. |
| LogP | 1.628 (est) |
| CAS DataBase Reference | 103-80-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzeneacetyl chloride(103-80-0) |
| EPA Substance Registry System | Benzeneacetyl chloride (103-80-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H290-H314-H335 | |||||||||
| Precautionary statements | P234-P261-P271-P280-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 34-37-14 | |||||||||
| Safety Statements | 26-36/37/39-45-25-27 | |||||||||
| RIDADR | UN 2577 8/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29163900 | |||||||||
| NFPA 704 |
|
Phenylacetyl chloride price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | P16753 | Phenylacetyl chloride 98% | 103-80-0 | 5G | ₹2381.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | P16753 | Phenylacetyl chloride 98% | 103-80-0 | 5G | ₹2381.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | P16753 | Phenylacetyl chloride 98% | 103-80-0 | 100G | ₹3120 | 2022-06-14 | Buy |
| Sigma-Aldrich | P16753 | Phenylacetyl chloride 98% | 103-80-0 | 500G | ₹14537.98 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | P16753 | Phenylacetyl chloride 98% | 103-80-0 | 500G | ₹14537.98 | 2022-06-14 | Buy |
Phenylacetyl chloride Chemical Properties,Uses,Production
Chemical Properties
colourless liquid
Uses
Phenylacetyl chloride is used in the preparation of 1,2-diphenyl-ethanone by reaction with iodobenzene using tetrabutylammonium tetrafluoroborate as a phase transfer catalyst. It is widely used in the chemical industry for manufacturing dyes and pharmaceutical products. It plays an important role in the preparation of penicillin. Further, it is used to prepare esters for flavorings.
General Description
A colorless volatile liquid with a strong odor. Denser than water. Contact may severely irritate skin, eyes and mucous membranes. May be toxic by ingestion. May also be combustible.
Air & Water Reactions
Soluble in water. Fumes in air. Decomposes in water or steam to form very corrosive hydrogen chloride gas.
Reactivity Profile
Phenylacetyl chloride is incompatible with strong oxidizing agents, alcohols, bases (including amines). May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Phenylacetyl chloride Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| sheetalchemicals | +91-9820130159 +91-9820130159 | Maharashtra, India | 77 | 58 | Inquiry |
| Freesia Chemicals | +9102225689889 | Mumbai, India | 45 | 58 | Inquiry |
| Rivashaa Agrotech Biopharma Pvt. Ltd. | +91-26463395 +91-7926462688 | Gujarat, India | 1615 | 58 | Inquiry |
| ALS INDIA LIFE SCIENCES | +91-8977036379 +91-8977036379 | Hyderabad, India | 1879 | 58 | Inquiry |
| Lords Biotech Inc | +91-9867440367 +91-9867440367 | Maharashtra, India | 15 | 58 | Inquiry |
| Neeta Interchem | +91-9427723676 +91-9427723676 | Gujarat, India | 19 | 58 | Inquiry |
| Stratechem Pvt Ltd | +91-2266627509 +91-2266627509 | Maharashtra, India | 23 | 58 | Inquiry |
| Stratechem (I) Pvt. Ltd. (SIPL) | 91-22-66627509 | Maharashtra, India | 14 | 58 | Inquiry |
| Vyas Bio Life Sciences Pvt. Ltd | -65546373 | Hyderabad, India | 1213 | 58 | Inquiry |
| Rexara Universeus. | 91--9723818993 | Gujarat, India | 8 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| sheetalchemicals | 58 |
| Freesia Chemicals | 58 |
| Rivashaa Agrotech Biopharma Pvt. Ltd. | 58 |
| ALS INDIA LIFE SCIENCES | 58 |
| Lords Biotech Inc | 58 |
| Neeta Interchem | 58 |
| Stratechem Pvt Ltd | 58 |
| Stratechem (I) Pvt. Ltd. (SIPL) | 58 |
| Vyas Bio Life Sciences Pvt. Ltd | 58 |
| Rexara Universeus. | 58 |
103-80-0(Phenylacetyl chloride)Related Search:
1of4
chevron_right




