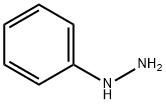
Phenylhydrazine
- CAS No.
- 100-63-0
- Chemical Name:
- Phenylhydrazine
- Synonyms
- Phenyl hydrazide;Fenilidrazina;phenyl-hydrazin;Phenyldiazane;JACS-100-63-0;Fenylhydrazine;Phenylhydrazin;PHENYLHYDRAZINE;HYDRAZINOBENZENE;AKOS BBS-00004362
- CBNumber:
- CB9216819
- Molecular Formula:
- C6H8N2
- Molecular Weight:
- 108.14
- MOL File:
- 100-63-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/11/28 16:31:44
| Melting point | 18-21 °C (lit.) |
|---|---|
| Boiling point | 238-241 °C (lit.) |
| Density | 1.098 g/mL at 25 °C (lit.) |
| vapor density | 4.3 (vs air) |
| vapor pressure | <0.1 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 192 °F |
| storage temp. | Store below +30°C. |
| solubility | Soluble in dilute acids. |
| form | Powder |
| pka | 8.79(at 15℃) |
| color | White to slightly blue or light beige |
| explosive limit | 1.1%(V) |
| Water Solubility | 145 g/L (20 ºC) |
| Sensitive | Air & Light Sensitive |
| Merck | 14,7293 |
| BRN | 606080 |
| Exposure limits | TLV-TWA skin 0.1 ppm (0.44 mg/m3) (ACGIH), 5 ppm (22 mg/m3) (OSHA); STEL 10 ppm (44 mg/m3) (OSHA); carcinogenicity: A2-Suspected Human Carcinogen (ACGIH), Carcinogen (NIOSH). . |
| Dielectric constant | 7.2(23℃) |
| Stability | Stable, but may decompose in sunlight. May be air or light sensitive. Incompatible with strong oxidizing agents, metal oxides. |
| CAS DataBase Reference | 100-63-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Hydrazine, phenyl-(100-63-0) |
| EPA Substance Registry System | Phenylhydrazine (100-63-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311+H331-H315-H317-H319-H341-H350-H372-H400 | |||||||||
| Precautionary statements | P273-P280-P301+P310-P302+P352+P312-P304+P340+P311-P305+P351+P338 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 45-23/24/25-36/38-43-48/23/24/25-50-68 | |||||||||
| Safety Statements | 53-45-61 | |||||||||
| OEL | Ceiling: 0.14 ppm (0.6 mg/m3) [2-hr] [skin] | |||||||||
| RIDADR | UN 2572 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | MV8925000 | |||||||||
| F | 8-10-23 | |||||||||
| Autoignition Temperature | 345 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2928 00 90 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 orally in Rabbit: 188 mg/kg | |||||||||
| IDLA | 15 ppm | |||||||||
| NFPA 704 |
|
Phenylhydrazine price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | P26252 | Phenylhydrazine 97% | 100-63-0 | 5G | ₹1948.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | P26252 | Phenylhydrazine 97% | 100-63-0 | 100G | ₹2067.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | P26252 | Phenylhydrazine 97% | 100-63-0 | 500G | ₹8844.03 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.07250 | Phenylhydrazine for synthesis | 100-63-0 | 5ML | ₹1790 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.07250 | Phenylhydrazine for synthesis | 100-63-0 | 250ML | ₹8720.01 | 2022-06-14 | Buy |
Phenylhydrazine Chemical Properties,Uses,Production
Chemical Properties
Phenylhydrazine is a colorless to pale yellow liquid or solid with a weak aromatic odor. It is soluble in water (values ranging from 145 to 837 g/litre at 24 °C have been reported) and is miscible with alcohol, ether, chloroform, benzene, and acetone. The conversion factor for phenylhydrazine is 1 ppm = 4.5 mg/m3 (at 20 °C, 101 kPa).
Physical properties
Yellow monoclinic crystals with a faint, aromatic odor. Turns red-brown on exposure to air.
Uses
Phenylhydrazine is used in the manufactureof dyes and pharmaceuticals; as a stabilizerfor explosive; as a reagent for aldehydes,ketones, and sugars in chemical analysis; andin organic synthesis.
Definition
ChEBI: A phenylhydrazine that is the monophenyl derivative of hydrazine.
General Description
Pale yellow crystals. Melting point 66°F. Becomes an oily liquid. Toxic by ingestion, inhalation and skin absorption. Flash point 192°F. Autoignition temperature 345°F. Soluble in alcohol.
Air & Water Reactions
When exposed to air becomes red-brown. Slightly denser than water and slightly soluble in water.
Reactivity Profile
Phenylhydrazine may ignite spontaneously when in contact with oxidants such as hydrogen peroxide or nitric acid, oxides of iron or copper, or manganese, lead, copper or their alloys. Will not polymerize [USCG, 1999].
Health Hazard
Phenylhydrazine is a moderate to highlytoxic compound and a carcinogen. Acutetoxic symptoms include hematuria, changesin liver and kidney, vomiting, convulsions,and respiratory arrest. Additional symptomsare lowering of body temperature and fallin blood pressure. Chronic exposure to thiscompound caused hemolytic anemia andsignificant body weight loss in rats. Anoral administration of 175 mg/kg was lethalto mice. An oral LD50 value in rat is188 mg/kg
Phenylhydrazine caused adverse repro ductive effect when dosed intraperitoneallyin pregnant mice. It caused jaundice, anemia,and behavioral deficit in offspring
Oral or subcutaneous administration ofphenylhydrazine or its hydrochloride produced lung and liver tumors in experimental animals. ACGIH lists this compoundas a suspected human carcinogen. The evidence of carcinogenicity of this compoundin human is inadequate.
Fire Hazard
Combustible liquid; flash point (closed cup) 89°C (192°F). It reacts with lead dioxide and strong oxidizing compounds vigorous to violently.
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Poison by ingestion, subcutaneous, and intravenous routes experimental reproductive effects Mutation data reported. Ingestion or subcutaneous injection can cause hemolysis of red blood cells. Other effects are damage to the spleen, liver, kidneys, and bone marrow. The most common effect of occupational exposure is the development of dermatitis, which, in sensitized persons, may be quite severe. Systemic effects include anemia and general weakness, gastrointestinal dsturbances, and injury to the kidneys. Flammable when exposed to heat, flame, or oxidizers. To fight fire, use alcohol foam. Violent reaction with 2phenylamino-3-phenyloxazirane. Reacts with perchloryl fluoride to form an explosive product. Vigorous reaction with lead(Ⅳ) oxide. Used as a chemical reagent, in organic synthesis, and in the manufacture of dyes and drugs. Dangerous; when heated to decomposition it emits highly toxic fumes of NOx; can react with oxidizing materials
Potential Exposure
Phenylhydrazine is a widely used reagent in conjunction with sugars, aldehydes, and ketones. In addition, to its use in the synthesis of dyes; pharmaceuticals, such as antipyrin; cryogenin, and pyramidone; and other organic chemicals. The hydrochloride salt is used in the treatment of polycythemia vera.
Carcinogenicity
Mice given 1mg phenylhydrazine orally each day, 7 days/week for 42 weeks had an increased incidence of total lung tumors. The incidence of malignant tumors also increased. In another study, mice administered 0.01% of the hydrochloride salt in drinking water (average of 0.63–0.81 mg/day) over their lifetimes had an increased incidence of blood vessel tumors. In contrast, mice given phenylhydrazine orally 5 days/week for 40 weeks at doses of 0.5 mg during the first 5 weeks and 0.25 mg thereafter failed to develop tumors.Higher doses could not be tested because of the marked anemia encountered. No leukemias occurred in mice treated with phenylhydrazine, and pulmonary tumors were infrequent . Oral administration or intraperitoneal injection (eight doses, each of 2.9 mg) of phenylhydrazine hydrochloride in mice failed to increase either pulmonary tumors or leukemia. The carcinogenic activity of hydrazine itself has been closely linked to its toxicity, that is, its irritant effect. The mechanism of action, it is postulated, is indirect alkylation of DNA, which is also closely connected to the toxic end point.
Shipping
UN2572 Phenylhydrazine, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Purify phenylhydrazine by chromatography, then crystallise it from pet ether (b 60-80o)/*benzene. Store it in the dark under N2 as it turns yellow, then red, on exposure to air. It is best stored as the hydrochloride salt; see below. [Coleman Org Synth Coll Vol I 442 1941, Shaw & Stratton J Chem Soc 5004 1962, Beilstein 15 IV 50.]
Incompatibilities
Phenylhydrazine is very reactive with carbonyl compounds, strong oxidizers; strong bases; alkali metals; ammonia, lead dioxide (violent). Attacks copper salts, nickel, and chromates.
Waste Disposal
Controlled incineration whereby oxides of nitrogen are removed from the effluent gas by scrubber, catalytic or thermal device.
Phenylhydrazine Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Seema Finechem Industry LLP | +91-8451840941 +91-8451840941 | NaviMumbai, India | 43 | 58 | Inquiry |
| Shree Sidhdhanath Industries | +91-9427163341 +91-9427163341 | Gujarat, India | 21 | 58 | Inquiry |
| Evans Fine Chem | +91-9821340302 +91-9821340302 | Maharashtra, India | 286 | 58 | Inquiry |
| WARUKSHA LABORATORIES PVT LTD | +91-7485988764 +91-7485988764 | Gujarat, India | 77 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Dhara Industries | +91-9322395199 +91-9322395199 | Mumbai, India | 190 | 58 | Inquiry |
| New Alliance Dye Chem Pvt Ltd | +91-9820112338 +91-9820112338 | Maharashtra, India | 104 | 58 | Inquiry |
| Excel Industries Limited | +91-22-66464200 +91-2266464200 | Mumbai, India | 17 | 58 | Inquiry |
| Vani Pharma Labs Limited | +91-4023193245 +91-4023193245 | Hyderabad, India | 17 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Seema Finechem Industry LLP | 58 |
| Shree Sidhdhanath Industries | 58 |
| Evans Fine Chem | 58 |
| WARUKSHA LABORATORIES PVT LTD | 58 |
| JSK Chemicals | 58 |
| Dhara Industries | 58 |
| New Alliance Dye Chem Pvt Ltd | 58 |
| Excel Industries Limited | 58 |
| Vani Pharma Labs Limited | 58 |
| Ralington Pharma | 58 |
Related articles
- Description, Synthesis and Usage of Phenylhydrazine
- Phenylhydrazine is used in the production of dyes, drugs, developer, etc. It is also an important reagent for identifying carb....
- Jul 11,2022





