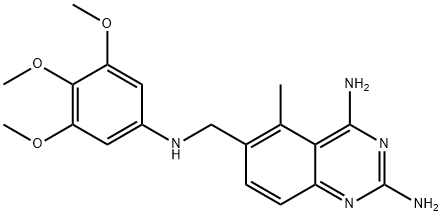
Trimetrexate
- CAS No.
- 52128-35-5
- Chemical Name:
- Trimetrexate
- Synonyms
- CI-898;NSC249008;NSC-249008;Trimetrexate;CI-898; CI 898; CI898;CI-898;NSC-249008;CI898;NSC249008;5-Methyl-6-(3,4,5-trimethoxyanilinomethyl)quinazoline-2,4-diamine;5-Methyl-6-[[(3,4,5-trimethoxyphenyl)amino]methyl]-2,4-quinazolinediamine;5-Methyl-6-{[(3,4,5-triMethoxyphenyl)aMino]Methyl}quinazoline-2,4-diaMine;2,4-Quinazolinediamine, 5-methyl-6-[[(3,4,5-trimethoxyphenyl)amino]methyl]-
- CBNumber:
- CB31075279
- Molecular Formula:
- C19H23N5O3
- Molecular Weight:
- 369.42
- MOL File:
- 52128-35-5.mol
- Modify Date:
- 2024/7/2 8:55:04
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
Trimetrexate Chemical Properties,Uses,Production
Description
Trimetrexate (TMQ) has been approved for the treatment of Pneumocystis carinii in patients with AIDS and also exhibits antiprotozoal activity against Trypanosoma cruzi . The drug is available as a single-ingredient medication, but it can be administered along with folinic acid in much the same way that methotrexate is administered with calcium leucovorin in cancer chemotherapy. Trimetrexate is a derivative of methotrexate.
Uses
Trimetrexate is an FDA-approved drug which selectively inhibits Streptococcus mutans through targeting dihydrofolate reductase (DHFR).
General Description
The drug is available as a lyophilized powder in 5- or 30-mgvials for IV use. The drug is used to treat colorectal cancer,head and neck cancer as well as NSCLC. The mechanism ofaction of trimetrexate involves folate antagonism and inhibitionof thymidylate synthesis. Trimetrexate does not formintracellular polyglutamate adducts as does methotrexateand other related compounds. Resistance can occur by increasedexpression of the target enzyme, decreased bindingaffinity for the target enzyme, or decreased intracellulardrug transport. Trimetrexate is administered only by the IVroute and distributed throughout the body with extensivebinding to plasma proteins. The major catabolic pathwaysinvolve O-demethylation followed by glucuronide conjugation.The drug interaction and toxicity profiles are similar tothose for methotrexate.
Mechanism of action
Trimetrexate is considered to be a nonclassical folate antagonist, whereas methotrexate, the structurally similar analogue of TMQ, is a classical folate antagonist. The difference between these two drugs is that methotrexate, with its polar glutamate side chain, is transported into the cell via a carrier-mediated transport system, whereas TMQ, without the glutamate moiety, is absorbed by the cell via a passive diffusion. Once in the cell, TMQ inhibits DHFR. Trimetrexate binds to Pneumocystis cari nii DHFR 1,500 times more strongly than trimethoprim and somewhat more strongly than methotrexate. It also has been reported that TMQ readily enters the P. carinii cell because of the lipophilic nature of this drug. Methotrexate and leucovorin are not able to enter the cell, however, because the cell membrane of P. carinii does not possess the transporter protein.
Clinical Use
Trimetrexate, when combined with the cytoprotective agent leucovorin, is more effective and better tolerated than pentamidine in the treatment of PCP. Because the first- and second-line agents are successful in only 50 to 75% of these cases, and because adverse reactions severely limit the use of some of the older agents, TMQ may offer some advantages in treatment. Trimetrexate is administered by IV infusion over 60 to 90 minutes and should be combined with the cytoprotective drug leucovorin. The leucovorin protects against bone marrow suppression and against renal and hepatic dysfunction. Leucovorin administration should continue for 72 hours after the last dose of TMQ. Additionally, TMQ has been reported to be effective in the treatment of Chagas' disease.
Trimetrexate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 | China | 4088 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | China | 9316 | 58 | Inquiry |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | China | 33350 | 58 | Inquiry |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 | China | 40067 | 58 | Inquiry |
| Amadis Chemical Company Limited | 571-89925085 | China | 131980 | 58 | Inquiry |
| Beijing Fubo Biotechnology Co., Ltd. | 010-57263844 13260236521 | CHINA | 6039 | 58 | Inquiry |
| Shanghai Han-Xiang Chemical Co., Ltd. | +86 (21) 5027-2946, +86 13818785766 | China | 1039 | 50 | Inquiry |
| Shanghai Benrui Biotechnology Co., Ltd. | 13917534498 | CHINA | 1472 | 58 | Inquiry |
| Shanghai Boyi Biotechnology Co., Ltd. | 021-58351080 15800446246 | CHINA | 5417 | 58 | Inquiry |
Related articles
- Side-effects of Trimetrexate
- Trimetrexate is a dihydrofolate reductase inhibitor used primarily as an alternative for treatment of the fungus Pneumocystis ....
- Mar 10,2022





