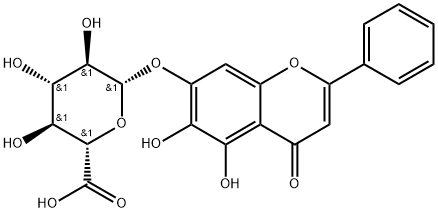
Baicalin
- CAS No.
- 21967-41-9
- Chemical Name:
- Baicalin
- Synonyms
- Radix;BAICALIN HYDRATE;Baicalein 7-O-glucuronide;Baicalein 7-O-β-D-glucuronide;5,6-Dihydroxy-4-oxygen-2-phenyl-4H-1-benzopyran-7-beta-D-glucopyranose acid;Bassora;Baicalin;Baicailin;Huang qin;BAICALIN(P)
- CBNumber:
- CB3111176
- Molecular Formula:
- C21H18O11
- Molecular Weight:
- 446.36
- MOL File:
- 21967-41-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/21 21:51:03
| Melting point | 202-205 °C (dec.) (lit.) |
|---|---|
| alpha | -85 º (c=1, DMSO) |
| Boiling point | 836.6±65.0 °C(Predicted) |
| Density | 1.737±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 2.72±0.70(Predicted) |
| form | solid |
| color | Pale Yellow to Yellow |
| optical activity | [α]20/D 85°, c = 1 in DMSO |
| Stability | Hygroscopic |
| InChIKey | IKIIZLYTISPENI-ZFORQUDYSA-N |
| SMILES | OC1C(=C(O[C@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](C(=O)O)O2)O)C=C2OC(C3C=CC=CC=3)=CC(=O)C=12)O |&1:5,6,7,9,11,r| |
| LogP | 1.431 (est) |
| CAS DataBase Reference | 21967-41-9(CAS DataBase Reference) |
Baicalin price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 572667 | Baicalin 95% | 21967-41-9 | 1G | ₹20935.55 | 2022-06-14 | Buy |
| TCI Chemicals (India) | B2835 | Baicalin | 21967-41-9 | 25G | ₹9900 | 2022-05-26 | Buy |
| ottokemi | B 1212 | Baicalin, 95%+ | 21967-41-9 | 1gm | ₹19602 | 2022-05-26 | Buy |
| ottokemi | B 1212 | Baicalin, 95%+ | 21967-41-9 | 5gm | ₹72099 | 2022-05-26 | Buy |
Baicalin Chemical Properties,Uses,Production
Description
Baicalin is one of the main active ingredients obtained from the roots of huang qin (Scutellaria baicalensis Georgi). The Pharmacopoeia of the People’s Republic of China (2015) stipulates that the content of baicalin in radix scutellariae with dry goods shall not be less than 9.0%. The medicinal radix scutellariae distribution in China are now Yunnan radix scutellariae (S. amoena C.?H. Wright), sticky hairs radix scutellariae (S. viscidula Bunge), Gansu radix scutellariae (S. rehderiana Diels), Lijiang radix scutellariae (S. likiangensis Diels), Sichuan radix scutellariae (S. hypericifolia Lev l.), radix scutellariae (S. tenax W.?W. Smith var. patentipilosa G.? Y. Wu), etc., which contain a certain amount of baicalin. Radix scutellariae medicinal has a long history in China. Listed as goods in Shen Nong’s Classic of Materia Medica, it has been used clinically to treat diseases with symptoms such as “heat jaundice, intestinal dysentery, edema, amenorrhea, malignant sore, and scleritis” for about 2000? years. Scutellaria is recorded in the Pharmacopoeia of the People’s Republic of China to have the effects of “clear heat and wet, purging fire to detoxify, stop bleeding and tocolysis.” It is one of the commonly used traditional Chinese herbs, which is clinically used alone or with other Chinese medicine compatibility for the treatment of respiratory infections, acute dysentery, viral hepatitis, allergic disease and gynecological disease, and so on.
Chemical Properties
Light yellow powder
Physical properties
Appearance: light yellow crystalline powder at room temperature. Solubility: insoluble in methanol, ethanol, and acetone; slightly soluble in chloroform and nitrobenzene; almost insoluble in water; soluble in hot acetic acid. Melting point: 202–205?°C.
History
The chemical research of the genus Scutellaria began in 1889. Baicalein (scutellarein) is the first flavonoid isolated from Vietnam radix scutellariae (S. altissima) in
1910. In 1922, Shibata Gui Tai and his collaborators isolated and obtained baicalin,
baicalein, wogonin, and benzoic acid from Scutellaria baicalensis.
Among the flavonoids in Scutellaria baicalensis, the content of baicalein is the
highest. Its official name is baikeli. Baicalin is formed by the combination of baicalein and one molecule of glucuronic acid. Both baicalein and baicalin exist in
Scutellaria baicalensis. Studies showed that baicalein could be transferred into
baicalin and other metabolites in the blood. However, baicalin by oral is hardly
absorbed. Baicalein can be absorbed and also rapidly converted into baicalin.
At present, there are a variety of mature extraction methods for obtaining baicalin
and baicalein from Scutellaria baicalensis. Due to the poor water solubility, oral
preparation of baicalin is mostly used in clinical practice. Research showed that
baicalin had a significant first pass effect, which led to its low bioavailability.
Baicalin-metal complexes formed by the combination of baicalin and metal ions
were found to enhance bioavailability and increase pharmacological activities.
In addition, the preparation of ester-type prednisone was easily hydrolyzed by esterase and could improve the lipid solubility of baicalin.
Uses
Baicalin has been used in a study to examine its neuroprotective effect in chronically stressed rats.2
Indications
Baicalin is mainly used for the adjuvant therapy of acute and chronic hepatitis and persistent hepatitis.
Definition
ChEBI: The glycosyloxyflavone which is the 7-O-glucuronide of baicalein.
General Description
Baicalin is a key flavonoid found in the roots of Scutellaria baicalensis. It is known to have different biological activities, such as anti-oxidative, anti-inflammatory, antitumor and anti-apoptotic activities.
Clinical Use
In clinical, baicalin is mainly used as the adjuvant therapy for acute and chronic
hepatitis and persistent hepatitis. Baicalin was shown to reduce the expression of
hepatitis B surface antigen, e antigen and the core antigen, and inhibited hepatitis B
virus DNA replication. Reduced serum alanine aminotransferase level was observed
in hepatitis patient treated with baicalin, and other liver function indexes were also
found to be improved. No adverse reactions about baicalin have been reported. In
addition, baicalin was reported to treat early diabetic nephropathy and alleviate the
symptoms of diabetic neuropathy
As one of the antimicrobial components of Scutellaria baicalensis, eye drops
containing 3% baicalin are used clinically in the treatment of trachoma, with the
similar curative effect as rifampicin.
Moreover, baicalein is used clinically for the treatment of enteritis and
dysentery.
Enzyme inhibitor
This baicalein glucuronide (FW = 446.36 g/mol; CAS 21967-41-9), also known as 5,6-dihydroxy-4-oxygen-2-phenyl-4H-1-benzopyran-7-b-D glucopyranose acid, is a flavone prodrug found in the Chinese medicinal herb Huang-chin (Scutellaria baicalensis) that is hydrolyzed to baicalein (See Baicalein). Baicalin is a slow, tight-binding inhibitor of Jack Bean urease, rapidly forming initial BA-urease complex (Ki = 3.9 × 10–3 M) that slowly isomerizes to the final complex (overall inhibition constant of Ki* = 0.15 × 10 μM. Inhibition can be reversed by dithiothreitol but not dilution of substrate. Baicalin also inhibits prolyl oligopeptidase, a cytosolic serine peptidase that hydrolyzes proline-containing peptides at the carboxy terminus of proline residues and is associated with schizophrenia, bipolar affective disorder, and related neuropsychiatric disorders. (See Pramiracetam)
Baicalin Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 32 | 58 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3889 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Chengdu ChenLv Herb Co.,Ltd | +undefined13608205856 | China | 127 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | China | 12460 | 58 | Inquiry |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 | China | 294 | 58 | Inquiry |
| Wuhan Haorong Biotechnology Co.,ltd | +86-18565342920; +8618565342920 | China | 293 | 58 | Inquiry |






