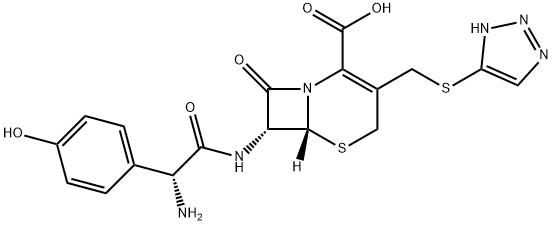
Cefatrizine
- CAS No.
- 51627-14-6
- Chemical Name:
- Cefatrizine
- Synonyms
- s640p;bls640;SKF-60771;eta(r)))-;CEFATRIZINE;cephatriazine;Cephathiamidine;antibioticbl-640;CEPHATHIAMIDINUM;antibioticbl-s640
- CBNumber:
- CB3116636
- Molecular Formula:
- C18H18N6O5S2
- Molecular Weight:
- 462.5
- MOL File:
- 51627-14-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/21 10:59:17
| Boiling point | 948.1±65.0 °C(Predicted) |
|---|---|
| Density | 1.3806 (rough estimate) |
| refractive index | 1.7000 (estimate) |
| storage temp. | Store at -20°C |
| solubility | DMSO: 95 mg/mL (201.02 mM);Ethanol: 23 mg/mL (48.67 mM) |
| pka | 2?+-.0.50(Predicted) |
| Water Solubility | Water: 95 mg/mL (201.02 mM) |
| CAS DataBase Reference | 51627-14-6(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Toxicity | LD50 in male, female mice, male, female rats (mg/kg): 6880, 6410, 4325, 4325 i.p. (Matsuzaki) |
|---|
Cefatrizine Chemical Properties,Uses,Production
Description
Cefatrizine was synthesized by BristolMyers Co. and Smith Klein & French Laboratories in 1974. It shows two to four times higher activity against gram-positive and four to eight times higher activity against gram-negative bacteria than cephalexin. Cefatrizine also shows excellent oral absorption, and its in vivo activity is 30 to 500 times higher than that of cephalexin.
Uses
Cefatrizine is a new orally administered cephalosporin with excellent activity against gram-positive cocci, inhibiting all except enterococci at minimal inhibitory concentrations below 1 μg/ml.
Definition
ChEBI: A cephalosporin compound having (1H-1,2,3-triazol-4-ylsulfanyl)methyl and [(2R)-2-amino-2-(4-hydroxyphenyl)]acetamido side-groups. An antibacterial drug first prepared in the 1970s, it has more recently been found to be n inhibitor of eukaryotic elongation factor-2 kinase (eEF2K), which is known to regulate apoptosis, autophagy and ER stress in many types of human cancers.
Antimicrobial activity
A semisynthetic cephalosporin formulated for oral use. The
spectrum is similar to that of cefalexin but it is more active
against H. influenzae. Wide strain variations in
susceptibility have been reported.
It is only partially absorbed when given by mouth. A
500 mg oral dose achieves a concentration of c. 6 mg/L after
1–2 h. The normal half-life of 2.5 h is extended to 5.5 h
in end-stage renal failure. Distribution resembles that of
cefalexin. It crosses the placenta readily. Plasma protein
binding is 40–60%.
Urinary recovery in 6 h is 35% of an oral dose, producing
urinary levels of 50–1500 mg/L. It is presumed that the
remainder is metabolized, but no metabolites have been
identified.
Apart from some mild diarrhea, it is well tolerated. It is
available in Japan.
Safety Profile
Moderately toxic byintraperitoneal and subcutaneous routes. An experimentalteratogen. Other experimental reproductive effects. Whenheated to decomposition it emits very highly toxic fumesof NOx and SOx.
Cefatrizine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| OCEAN TRADING CORPORATION | +91(22) 24921669 | New Delhi, India | 6211 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | China | 6387 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| SIMAGCHEM CORP | +86-13806087780 | China | 17367 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
51627-14-6(Cefatrizine)Related Search:
1of3
chevron_right




