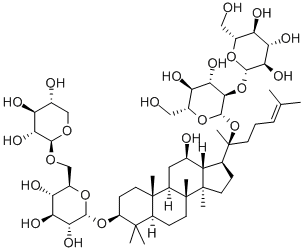
20(R)-Ginsenoside Rg3
- CAS No.
- 38243-03-7
- Chemical Name:
- 20(R)-Ginsenoside Rg3
- Synonyms
- Ginsenoside Rg3 (R-Form);CID 9918693;dNTP Solution;Ginsenoside Rg;Ginseonoside Rg3;20(r)-propanaxadiol;Ginsenoside Rg3 Rh2;Ginsenosides rg3(R);Ginsenoside Rg3 (R-);20(R)-Ginsenoside Rg3
- CBNumber:
- CB42130576
- Molecular Formula:
- C42H72O13
- Molecular Weight:
- 785.01
- MOL File:
- 38243-03-7.mol
- Modify Date:
- 2024/7/8 20:25:11
| Melting point | 315-318 ºC |
|---|---|
| Boiling point | 885.0±65.0 °C(Predicted) |
| Density | 1.30 |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Very Slightly, Heated Sonicated), Pyridine (Slightly, Heated, Sonicated) |
| form | Solid |
| pka | 12.85±0.70(Predicted) |
| color | White to Off-White |
| Stability | Hygroscopic |
| InChIKey | VMFHKVMQSYOBAV-KQVQOVSDNA-N |
20(R)-Ginsenoside Rg3 Chemical Properties,Uses,Production
Description
Ginsenoside Rg3 is mainly derived from the dried roots of Araliaceae Panax ginseng C.A.?Mey. and Panax quinquefolius L.?Ginseng has a long history of medicinal uses, well known as “king of herbs.” Ginseng is listed as the top grade in “Shen Nong’s herbal class,” with “the main security set up the five internal organs, spirit, soul, stop fright, in addition to evil, eyesight, fun puzzle” account. The traditional Chinese medicine regards ginseng as the top grade of reinforcing Qi, with Dabu strength, spleen and lungs, Sheng Jin, Anshen Yizhi, etc.
History
Ginseng is currently one of the most studied traditional medicines. Chemical researches certificate that ginseng contains a variety of ginseng saponin, ginseng polysaccharide, protein, polypeptide, amino acids, and other chemical constituents. Ginseng saponins are the main active components, accounting for about 4%. Ginsenoside is a kind of sterol compound, which has a similar basic structure. Ginsenoside contains the steroid nucleus of 17 carbon atoms arranged in 4 rings. At present, more than 40 kinds of ginsenosides have been isolated from ginseng. They are divided into four types according to their chemical structure, the protopanaxadiol, the protopanaxatriol, the ocotillol, and the oleanolic acid.
Ginsenoside Rg3 is a dammarane tetracyclic triterpenoid saponin found in natural ginseng, which belongs to the protopanaxadiol . It is also the natural ingredient with prominent activity extracted from red ginseng (processed ginseng). Modern
medicine has proved that Rg3 has certain biological activity. Since 1983, Rg3 has
been widely studied by many scholars in the world . The results showed that the
content of ginsenoside Rg3 in the fresh and dried ginseng roots was less than 1 ppm
while in red ginseng was 3/100,000. After several years of research, the method of
extracting 20(R)-ginsenoside Rg3 from red ginseng has been successfully improved,
and the industrialized production has been realized. A multicenter clinical study by
eight domestic hospitals indicated that 20(R)-ginsenoside Rg3 has therapeutic
effects on tumor. It has been issued the new drug certificate by the Chinese Food and
Drug Administration, as a new anticancer drug approved.
Uses
20(R)-Ginsenoside Rg3 causes an inhibition of phosphatidylinositol 3-kinase/Akt activation that, in turn, results in modulations in Bcl-2 family proteins in such a way that the apoptosis of U87 cells are regulated. Elicits a significant inhibition of in vitro cell adhesion and invasion of U87 cells. in vitro stereoselective inhibition of ginsenosides toward UDP-glucuronosyltransferase isoforms and in vivo inhibition of tumor angiogenesis of lung cancer.
Indications
This product is contained in “New drug standards” the 87th volume.
Ginsenoside Rg3 capsule, ginsenoside Rg3 emulsion, and ginsenoside Rg3 microemulsion are mainly used in the clinical treatment or adjuvant therapy of lung cancer, breast cancer, liver cancer, gastric cancer, colon cancer, etc. In addition, they improve and prevent the common diseases in the elderly such as cardiovascular disease, coronary heart disease, limb weakness, difficulty walking, and loss of memory. Ginsenoside Rg3 also has a certain biological activity in antiviral, radiosensitization, and treatment of chronic obstructive pulmonary disease.
Definition
Ginsenoside Rg3 is a tetracyclic triterpenoid saponin type rich in red ginseng. The major ginsenosides, such as Rb1, Rb2, and Rg3. Due to the different spatial structures from C20 positions, there are two enantiomers, 20(R) and 20(S)-isomers. With differential conguration, their antitumor activities exhibit certain differences. 20(R)-ginsenoside Rg3 and 20(S)-ginsenoside Rg3 can inhibit tumor metastasis by the suppression of EMT. 20(R)-ginsenoside Rg3 suppressed lung cancer migration or/and invasion by inhib-iting TGF-β1-induced EMT accompanied by the inactivation of MMP-2, p38 MAPK and Smad2. In addition, 20(S)-ginsenoside Rg3 effectively suppressed hypoxia-induced EMT, inhibiting ovarian cancer metastasis[1].
Pharmacology
It is reported that the antitumor effects of ginsenoside Rg3 and Rh2 were the most significant in more than 40 kinds of ginsenosides obtained. Moreover, they enhanced the immune function of tumor patients after chemotherapy, including humoral immunity, cell immunity, and non-specific immunity . This effect is consistent with the vitality nourishing and body strengthening of ginseng in the theory of traditional Chinese medicine. There is more research on the effects of 20(R)-ginsenoside Rg3?in the two isomers of ginsenoside Rg3, which has certain inhibitory effects on tumor cells of lung cancer, gastric cancer, colon cancer, breast cancer, liver cancer, etc. There is less research on the effects of 20(S)-ginsenoside Rg3. Other studies have shown that ginsenoside Rg3 also has the effects of anti-fatigue , antiviral , and treatment of chronic obstructive pulmonary disease .
Human tolerance test shows that ginsenoside Rg3 has a wide safety range. The
recommended dose of the first phase of clinical trial (0.8 mg/kg/day) has no side
effects. Ginsenoside Rg3 has good oral absorption. The drug absorption peak in the
blood is detected after administration for 15–30 min, and the blood concentration
reaches the peak after administration for 1.0–1.5 h. The half-life is 4.84±1.41 h. The maximum blood concentration increases with the dose within the reagent range
(0.8–3.2 mg/kd/day), suggesting the pharmacokinetics of ginsenoside Rg3 the firstorder kinetics process.
References
[1] Central Laboratory. “Anticancer effects of ginsenoside Rg3 (Review).”International Journal of Molecular Medicine 39 3 (2017): 507-518.
20(R)-Ginsenoside Rg3 Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Chengdu Biopurify Phytochemicals Ltd. | +86-28-82633860 +86-18080483897 | China | 3424 | 58 | Inquiry |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +86-18580541567 +86-17782035140 | China | 301 | 58 | Inquiry |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | CHINA | 1824 | 58 | Inquiry |
| Xi'an Kono chem co., Ltd., | 029-86107037 13289246953 | China | 2995 | 58 | Inquiry |
| Shaanxi Pioneer Biotech Co., Ltd . | +8613259417953 | China | 3000 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| Shanghai Longyu Biotechnology Co., Ltd. | +8613917842738 | China | 2534 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
38243-03-7(20(R)-Ginsenoside Rg3)Related Search:
1of4
chevron_right




