ARSINE
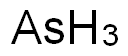
- CAS No.
- 7784-42-1
- Chemical Name:
- ARSINE
- Synonyms
- AsH3;ARSINE;ARSENE;Arsenowodor;Arsenic hydrid;INORGANICARSINE;Arsenic hydride;Arsenwasserstoff;Arsenous hydride;Hydrogen arsenide
- CBNumber:
- CB4375809
- Molecular Formula:
- AsH3
- Molecular Weight:
- 77.94542
- MOL File:
- 7784-42-1.mol
- Modify Date:
- 2024/3/14 15:18:26
| Melting point | -117° |
|---|---|
| Boiling point | bp -62.5° |
| Density | 1.321 (estimate) |
| vapor pressure | >760 mmHg at 20 °C |
| solubility | slightly soluble in H2O |
| form | colorless gas |
| color | colorless |
| Odor | Garlic-like odor detectable at 0.5 to 1 ppm |
| Water Solubility | mL/100g H2O (760mm): 42 (0°C), 30 (10°C), 28 (20°C) [LAN05] |
| Exposure limits | TLV-TWA 0.2 mg/m3 (0.05 ppm) (ACGIH and OSHA); 0.002 mg(As)/m3/15 min; ceiling 0.005 ppm(As)/15 min (NIOSH). |
| Dielectric constant | 2.5(-100℃) |
| Stability | Stable, but pyrophoric. Capable of detonation with some initiators. Flammable. Incompatible with oxidizing agents, chlorine, nitric acid. |
| EPA Substance Registry System | Arsine (7784-42-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |      GHS02,GHS04,GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H220-H330-H373-H410 | |||||||||
| Precautionary statements | P210-P377-P381-P403-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501-P260-P314-P501-P273-P391-P501 | |||||||||
| Hazard Codes | F+,T+,N | |||||||||
| Risk Statements | 12-26-48/20-50/53 | |||||||||
| Safety Statements | 9-16-28-33-36/37-45-60-61 | |||||||||
| RIDADR | 2188 | |||||||||
| OEL | Ceiling: 0.002 mg/m3 [15-minute] | |||||||||
| Autoignition Temperature | Not established. Decomposes at 232 to 300 °C to form elemental arsenic and hydrogen. | |||||||||
| DOT Classification | 2.3, Hazard Zone A (Gas poisonous by inhalation) | |||||||||
| HazardClass | 2.3 | |||||||||
| Toxicity | LCLO inhal (rat) 94 ppm (300 mg/m3; 15 min) PEL (OSHA) 0.05 ppm (0.2 mg/m3) TLV-TWA (ACGIH) 0.05 ppm (0.16 mg/m3) |
|||||||||
| IDLA | 3 ppm | |||||||||
| NFPA 704 |
|
ARSINE Chemical Properties,Uses,Production
Description
Arsine is a colorless, extremely toxic, flammable
gas at room temperature and atmospheric
pressure and is heavier than air. It has a mild
garliclike odor and acts as a blood and nerve
poison. It can be fatal if inhaled in sufficient
quantity and can form flammable mixtures with
air.
Arsine is shipped as a liquefied compressed
gas in steel cylinders under its own vapor pressure
of 219.7 psia (1515 kPa, abs). Arsine is
slightly soluble in both water and organic solvents.
It reacts readily with agents such as potassium
permanganate, bromine, and sodium
hypochlorite to form arsenic compounds. Arsine
is stable at room temperature, but begins to decompose
into its elements around 446°F to
464°F (230°C to 240°C).
Chemical Properties
Arsine is a highly toxic, colorless gas with a garlic odor. It is soluble in water, benzene, and chloroform. It is extremely flammable and explosive when exposed to heat, sparks, or flames. Arsine decomposes on heating and under the infl uence of light and moisture, producing toxic arsenic fumes. Arsine reacts with strong oxidants, causing explosion hazard and may explosively decompose on shock, friction, or concussion. Workers in the metallurgical industry involved in the production process and the maintenance of furnaces, and in the microelectronics industry get exposed to the substance. Arsine is extensively used in semiconductor industries, and in the manufacture of microchips.
Physical properties
Colorless gas; garlic-like unpleasant odor; liquefies at -55°C; solidifies at -116.3°C; heavier than air; gas density 2.695 (air =1); sparingly soluble in cold water (~ 20 mg/100 g water or about 640 mg/L at the NTP); soluble in chloroform and benzene.
Uses
Arsine (AsH3), as a colorless gas, is also known as arsenic hydride. It is used to synthesize organic compounds and as the major ingredient of several military poisons, including the wartime gas lewisite.
Preparation
Arsine is produced by the reaction of arsenic trichloride, arsenic trioxide or
any inorganic arsenic compound with zinc and sulfuric acid. It is also made by
treating a solution of sodium arsenide or potassium arsenide in liquid ammonia
with ammonium bromide:
Na3As + 3NH4Br → AsH3 + 3NaBr + 3NH3
It may be also prepared by decomposition of alkali metal arsenides by water;
or arsenides of other metals with acids:
Ca3As2 + 6 HCl → 2 AsH3 + 3 CaCl2
A poor yield may be obtained if water is substituted for acids. Thus calcium arsenide reacts with water to produce about 15% arsine.
Definition
A poisonous colorless gas with an unpleasant smell. It decomposes to arsenic and hydrogen at 230°C. It is produced in the analysis for arsenic (Marsh’s test).
Air & Water Reactions
Highly flammable. On exposure to light, decomposes rapidly depositing shiny black arsenic.
Reactivity Profile
ARSINE decomposes into its elements (arsenic, gaseous hydrogen) when heated to 300°C. Can form accidentally by the reaction of arsenic impurities with mineral acids (hydrochloric acid, sulfuric acid) in the presence of common metals (iron, zinc). A reducing agent---not oxidized by air at room temperature [Kirk-Othmer, 3rd ed., Vol. 3, 1978, p. 251], but may react vigorously with other oxidizing agents [Sax, 9th ed., 1996, p. 279]. Moderately explosive in combination with chlorine or nitric acid. When heated to decomposition or ignited, ARSINE emits highly toxic fumes of metallic arsenic.
Hazard
Highly poisonous by inhalation. Periph- eral nervous system and vacular system impairment, kidney and liver impairment.
Health Hazard
ARSINE is highly toxic by inhalation; a very short exposure to small quantities may cause death or permanent injury. ARSINE is the most powerful hemolytic poison encountered in industry.
Fire Hazard
Vapors may travel to a source of ignition and flash back. Container may explode in heat of fire. When heated to decomposition, emits highly toxic fumes. Can react vigorously with oxidizing materials. May explode when exposed to chlorine, nitric acid, or potassium plus ammonia. On exposure to light, moist ARSINE decomposes quickly, depositing shiny black arsenic.
Flammability and Explosibility
Arsine is flammable in air, having a lower explosion limit (LEL) of 5.8%. The upper limit has not been determined. Combustion products (arsenic trioxide and water) are less toxic than arsine itself. In the event of an arsine fire, stop the flow of gas if possible without risk of harmful exposure and let the fire burn itself out.
Materials Uses
Arsine is noncorrosive and may, therefore, be used with most of the commercially available metals. However, since arsine is mainly used for the electronics industry, stainless steel is recommended for the gas delivery systems. Stainless steel regulators should be used for all highpurity applications with arsine and arsine mixtures.
Safety Profile
Confirmed human carcinogen. Poison by inhalation. Human red blood cell, gastrointestinal system, central nervous system, and other systemic effects by inhalation. Flammable when exposed to flame. Moderately explosive when exposed to Cl2, HNO3, (K + NH3, open flame, or powerful shock. Dangerous, more toxic than its oxidation product. When heated to decomposition it emits highly toxic fumes of arsenic. See also ARSENIC, ARSENIC COMPOUNDS, and HYDRIDES.
Potential Exposure
Arsine is used in making electronic, semiconductor components; in organic syntheses; and in making lead-acid storage batteries. Arsine may be generated by side reactions or unexpectedly; e.g., it may be generated in metal pickling operations; metal drossing operations; or when inorganic arsenic compounds contact sources of nascent hydrogen. It has been known to occur as an impurity in acetylene. Most occupational exposure occurs in chemical, smelting, and refining industries. It has been used as a poison gas. Cases of exposure have come from workers dealing with zinc, tin, cadmium, galvanized coated aluminum; and silicon and steel metals. A regulated, marked area should be established where this chemical is handled, used, or stored in compliance with OSHA Standard 1910.1045. SA is used as a military poison gas (blood agent). It forms cyanide in the body.
Carcinogenicity
Arsenic has been considered a human carcinogen for a number of years(1),but the mechanisms underlying these processes have remained elusive due in part to the absence of an appropriate animal model. There are a number of hypotheses for the mechanisms of arsenical action that include arsenical inhibition of DNA repair, cocarcinogenesis, and more recently the concept of arsenical production of ROS(65,66) that may act in concert with these mechanisms. It is clear from in vitro mutagenicity test systems that arsenicals are not direct-acting mutagens but rather act via some secondary mechanism(s). Given the long history and knowledge that arsenicals in air and water produce human cancers, this is a remarkable situation with regard to occupational and environmental exposures. Most studies of animals exposed to arsenate or arsenite by the oral route have not detected any clear evidence for an increased incidence of skin cancer or other cancers. Recently, a series of studies presented evidence that inorganic arsenic may be a transplacental carcinogen in animals. Waalkes et al. exposed timed pregnant mice to sodium arsenite in drinking water during gestation days 8–18. Dose-related increases in hepatocellular carcinomas and adrenal tumors in the male offspring and uterine hyperplasia in female offspring from treated dams were reported. The offspring also had increase in the number of malignant tumors. Aberrant estrogen signaling, potentially through inappropriate estrogen receptora, may play a role in the arsenic-induced tumors in these offspring.
Environmental Fate
Arsine acts predominantly as a hemolytic agent. Hemolysis appears to be dependent on membrane disruption as a result of arsine reactions with sulfhydryl groups and from formation of hydrogen peroxide and adducts with oxyhemoglobin. Failure of the kidneys and other organs is probably not only due to the effects of red blood cell debris slugging within the microcirculation but also to a direct toxic effect on the organs.
storage
cylinders of arsine should be stored and used in a continuously ventilated gas cabinet or fume hood. Local fire codes should be reviewed for limitations on quantity and storage requirements. Carbon steel, stainless steel, Monel?, and Hastelloy ?C are preferred materials for handling arsine; brass and aluminum should be avoided. Kel-F? and Teflon? are preferred gasket materials; Viton? and Nylon? are acceptable.
Shipping
UN2188 Arsine, Hazard class: 2.3; Labels: 2.3- Poisonous gas, 2.1-Flammable gas, Inhalation Hazard Zone A. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner. Military driver shall be given full and complete information regarding shipment and conditions in case of emergency. AR 50-6 deals specifically with the shipment of chemical agents. Shipments of agent will be escorted in accordance with AR 740-32.
Incompatibilities
Arsine forms explosive mixture with air. SA reacts with strong oxidizers, nitric acid, causing an explosion hazard. Thermally unstable; shock, friction, and concussion sensitive; can explosively decompose. Can explode on contact with warm, dry air. Violent reaction with acids, halogens, mixtures of potassium and ammonia. Decomposes to metallic arsenic (fumes) on exposure to light, moisture or upon decomposition from heat or ignition.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Arsine may be disposed of by controlled burning. When possible, cylinders should be sealed and returned to suppliers. Seek guidance from regulatory agencies as to proper disposal. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Precautions
Occupational workers should be careful during handling/use of arsine. Workers should use protective gloves: neoprene, butyl rubber, PVC, polyethylene, or Tefl on. Workers should also use appropriate protective equipment. If a leak occurs in a user’s equipment, be certain to purge the piping with an inert gas prior to attempting repairs and evacuate all personnel from the affected area. The compressed gas cylinders should not be refi lled without the express written permission of the owner.
ARSINE Preparation Products And Raw materials
Raw materials
Preparation Products
7784-42-1(ARSINE)Related Search:
1of4
chevron_right




