Gallium arsenide
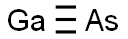
- CAS No.
- 1303-00-0
- Chemical Name:
- Gallium arsenide
- Synonyms
- Lead(Pb);GALLIUM ARSENIDE;Arsinidynegallium;galliummonoarsenide;gallanylidynearsane;galliumarsenide(gaas);galliumarsenide[gaas];gallium(III) arsenide;Gallium arsenide wafer;Gallium Arsenide Powder
- CBNumber:
- CB7249244
- Molecular Formula:
- AsGa
- Molecular Weight:
- 144.64
- MOL File:
- 1303-00-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/11/14 16:42:34
| Melting point | 1238°C |
|---|---|
| Density | 5.31 g/mL at 25 °C (lit.) |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 3.57 |
| form | pieces |
| color | Dark gray |
| Specific Gravity | 5.31 |
| Resistivity | ≥1E7 Ω-cm |
| Water Solubility | Soluble in hydrochloric acid. Insoluble in water, ethanol, methanol and acetone. |
| Crystal Structure | Cubic, Sphalerite Structure - Space Group F(-4)3m |
| Merck | 14,4347 |
| CAS DataBase Reference | 1303-00-0(CAS DataBase Reference) |
| IARC | (Vol. 86, 100C) 2012 |
| EPA Substance Registry System | Gallium arsenide (1303-00-0) |
| Knoop Microhardness | 7500, N/mm2 |
|---|
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H350-H360F-H372 | |||||||||
| Precautionary statements | P202-P260-P264-P270-P280-P308+P313 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 23/25-50/53-48/23-45 | |||||||||
| Safety Statements | 20/21-28-45-60-61-53 | |||||||||
| RIDADR | UN 1557 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LW8800000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 2853909090 | |||||||||
| Toxicity | mouse,LD50,intraperitoneal,4700mg/kg (4700mg/kg),BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE)BEHAVIORAL: FOOD INTAKE (ANIMAL),Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 45(10), Pg. 13, 1980. | |||||||||
| NFPA 704 |
|
Gallium arsenide price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 651486 | Gallium arsenide (single crystal substrate), <100>, diam. × thickness 2?in. × 0.5?mm | 1303-00-0 | 1EA | ₹44967.05 | 2022-06-14 | Buy |
| ALFA India | ALF-088458-18 | Gallium arsenide, 99.999% (metals basis) | 1303-00-0 | 50g | ₹127835 | 2022-05-26 | Buy |
| ALFA India | ALF-088458-09 | Gallium arsenide, 99.999% (metals basis) | 1303-00-0 | 10g | ₹64880 | 2022-05-26 | Buy |
| ALFA India | ALF-088458-04 | Gallium arsenide, 99.999% (metals basis) | 1303-00-0 | 2g | ₹10227 | 2022-05-26 | Buy |
| ottokemi | GSC 111 | Gallium arsenide (GaAs substrate), <100>, (undoped, 10 x 10 x 0.5 mm) | 1303-00-0 | 1pc | ₹207099 | 2022-05-26 | Buy |
Gallium arsenide Chemical Properties,Uses,Production
Chemical Properties
Gallium arsenide contains 48.2% gallium and 51.8% arsenic and is considered an intermetallic compound. It occurs as cubic crystals with a dark gray metallic sheen. Gallium arsenide is electroluminescent in infrared light. Gallium arsenide readily reacts with oxygen in air, forming a mixture of oxides of gallium and arsenic on the crystal surface. Gallium arsenide presents the following properties: hardness, 4.5; thermal expansion coefficient, 5.9×106; thermal conductivity, 0.52Wunits; specific heat, 0.086 cal/g/ ℃; intrinsic electron concentration, 107; energy gap at room temperature, 1.38 eV; electron mobility, 8800 cm2/V/s; effective mass for electrons, 0.06m0; lattice constant,5.6–54 AO; dielectric constant, 11.1; intrinsic resistivity at 300K=3.7×108Ωcm; electron lattice mobility at 300K= 10,000 cm2/V/s; intrinsic charge density at 300K=1.4 106 cm-3; electron diffusion constant at 300K=310 cm2/s; and hole diffusion constant=11.5 cm2/s.

Garlic odor when moistened. Finely divided gallium arsenide can react vigorously with steam, energetic acids, and oxidizers to evolve arsine gas, and can release arsenic fumes when heated to decomposition. The molten form attacks quartz.
Physical properties
Gray cubic crystal; density 5.316 g/cm3; melts at 1,227°C; hardness 4.5 Mohs; lattice constant 5.653?; dielectric constant 11.1; resistivity (intrinsic) at 27°C, 3.7x108 ohm-cm.
Uses
In semiconductor applications (transistors, solar cells, lasers).Gallium arsenide is among the most widely used intermetallic semiconductor components (Harrison, 1986; McIntyr and Sherin, 1989). Gallium arsenide is also incorporated into light-emitting diodes and photovoltaic cells, while gallium alloys are used for dental amalgam as a low toxicity replacement for mercury.
Uses
Gallium arsenide is electroluminscent in infrared light and is used for telephone equipment, lasers, solar cell, and other electronic devices. GaAs is used as the substrate of the infrared low-pass filter.
Preparation
Gallium arsenide is prepared by passing a mixture of arsenic vapor and hydrogen over gallium(III) oxide heated at 600°C: Ga2O3 + 2As + 3H2 2GaAs + 3H2OThe molten material attacks quartz. Therefore, quartz boats coated with carbon by pyrolytic decomposition of methane should be used in refining the compound to obtain high purity material. Gallium arsenide is produced in polycrystalline form as high purity, single crystals for electronic applications. It is produced as ingots or alloys, combined with indium arsenide or gallium phosphide, for semiconductor applications.
Production Methods
Gallium (Ga) and arsenic (As), heated in a vacuum to eliminate oxygen, are filled in the silica boat
and the boat is vacuum-sealed in a silica tube. A single crystal is obtained by putting the silica tube
into the horizontal Stockbarger furnace with three zones: A (605℃), B (1250℃) and C (1100℃),
and by transporting the tube in the direction A→B→C with the speed of 2 cm/h. The Czochralski
method can also be used (refer to InAs).
The vapor phase method can be used to deposit the thin films. For instance, the GaAs single
crystal is grown on the low temperature area by heating the closed tube filled with GaAs together
with I2, Cl2, or HCl gas with a temperature gradient. Using this method, we can grow the
epitaxial layer.
General Description
Dark gray crystals with a metallic greenish-blue sheen or gray powder. Melting point 85.6°F (29.78°C).
Air & Water Reactions
Stable in dry air. Tarnishes in moist air. Insoluble in water.
Reactivity Profile
GALLIUM ARSENIDE can react with steam, acids and acid fumes. Reacts with bases with evolution of hydrogen. Attacked by cold concentrated hydrochloric acid. Readily attacked by the halogens. The molten form attacks quartz.
Hazard
Toxic metal. Questionable carcinogen
Fire Hazard
Flash point data for GALLIUM ARSENIDE are not available; however, GALLIUM ARSENIDE is probably combustible.
Safety Profile
Confirmed carcinogen. Mddly toxic by intraperitoneal route. Most arsenic compounds are poisons. Can react with steam, acids, and acid fumes to evolve the deadly poisonous arsine. Molten gallium arsenide attacks quartz. When heated to decomposition it emits very toxic fumes of As. See also ARSENIC COMPOUNDS and GALLIUM COMPOUNDS.
Carcinogenicity
Carcinogenesis.
Gallium is antineoplastic in several
human and murine cancer cell lines and in some in vivo
cancers. It has been used experimentally in patients in the
treatment of lymphatic malignancies (including multiple
myelomas) and for urothelial malignancies. However,
the dissociation of gallium arsenide into gallium and arsenic
is a factor to take into account. A considerable number of
studies have evaluated the carcinogenic potential of arsenic
and various arsenic compounds.
Structure and conformation
The space lattice of gallium arsenide (GaAs) belongs to the cubic system Td2, and its zincblende-type structure has a lattice constant of a=0.5654 nm and a distance to its nearest neighbor of 0.244 nm.
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| Aritech Chemazone Private Limited | +91-9034345475 | Punjab, India | 684 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| SINAXIS ENTERPRISES PRIVATE LIMITED | 08048372738Ext 355 | Mumbai, India | 1 | 58 | Inquiry |
| Nano Research Elements | 08048372588Ext 365 | Delhi, India | 101 | 58 | Inquiry |
| Intelligent Materials Private Limited | 08048953178 | Punjab, India | 197 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21677 | 55 | Inquiry |
| career henan chemical co | +86-0371-86658258 15093356674; | China | 29902 | 58 | Inquiry |
Related articles
- Crystal Structure of Gallium arsenide
- Gallium arsenide (GaAs) is a III-V compound semiconductor material. It consists of the metal atoms gallium (Ga) and arsenic (A....
- Jun 14,2024
1303-00-0(Gallium arsenide)Related Search:
1of4
chevron_right




