ALAMETHICINRESEARCH GRADE
- CAS No.
- 27061-78-5
- Chemical Name:
- ALAMETHICINRESEARCH GRADE
- Synonyms
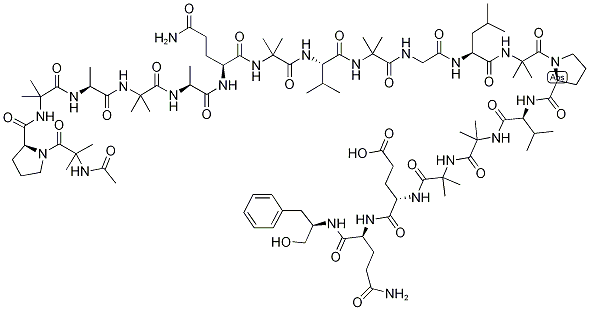
- Alamethicin (U-22324);ALAMETHICINRESEARCH GRADE;Alamethicin, Ready Made Solution;alamethicin from trichoderma viride;Alamethicin, Ready Made Solution from Trichoderma viride;Peptaibol coMplex. Additional synonyMs for the Major coMponent, alaMethicin I: AlaMethicin F30, AlaMethicin-gaMMa, U 22324;Ac-2-MeAla-L-Pro-2-MeAla-L-Ala-2-MeAla-L-Ala-L-Glu(NH2)-2-MeAla-L-Val-2-MeAla-Gly-L-Leu-2-MeAla-L-Pro-L-Val-2-MeAla-2-MeAla-L-Glu-L-Glu(NH2)-phenylalaninol;Almethicin, Antibiotic U-22324, 6-(2-methylalanine)-8-L-leucine-9-de-L-valine-12-(2-methylalanine)-13a-endo-(2-methylalanine)-18-L-glutamine-19-(N(1)-(1-(hydroxymethyl)-3-methylbutyl)-L-glutamamide)-];N-Acetyl-2-methylalanyl-L-prolyl-2-methylalanyl-L-alanyl-2-methylalanyl-L-alanyl-L-glutaminyl-2-methylalanyl-L-valyl-2-methylalanylglycyl-L-leucyl-2-methylalanyl-L-prolyl-L-valyl-2-methylalanyl-2-methylalanyl-L-α-glutamyl-N1;(3S,12R)-1-((S)-1-((6S,12S,15S,21S,30S)-1-((R)-1-(2-AcetaMido-2-Methylpropanoyl)pyrrolidin-2-yl)-15-(3-aMino-3-oxopropyl)-30-isobutyl-21-isopropyl-3,3,6,9,9,2,18,18,24,24,33,33-dodecaMethyl-1,4,7,10,13,16,19,22,25,28,31-undecaoxo-2,5,8,11,1
- CBNumber:
- CB4500700
- Molecular Formula:
- C92H150N22O25
- Molecular Weight:
- 1964.3078
- MOL File:
- 27061-78-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/1/18 18:09:09
ALAMETHICINRESEARCH GRADE price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | A4665 | Alamethicin from Trichoderma viride ≥98% (HPLC) | 27061-78-5 | 5MG | ₹24139.75 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | A5361 | Alamethicin, Ready Made Solution from Trichoderma viride 5?mg/mL in DMSO | 27061-78-5 | 500μL | ₹24312.4 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | A4665 | Alamethicin from Trichoderma viride ≥98% (HPLC) | 27061-78-5 | 10MG | ₹42888.65 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | A4665 | Alamethicin from Trichoderma viride ≥98% (HPLC) | 27061-78-5 | 25MG | ₹94069.25 | 2022-06-14 | Buy |
ALAMETHICINRESEARCH GRADE Chemical Properties,Uses,Production
Description
Alamethicin is an antibiotic peptide belonging to a class of membrane active peptides of fungal origin called peptaibols. It contains an unusual amphiphilic amino acid, 2-
Chemical Properties
solid
Uses
Alamethicin is an acidic linear peptaibol complex with potent antibiotic activity, containing 20 “amino acids”, with acetyl and phenylalaninol termini, produced by Trichoderma sp.. Alamethicin F30 acts an ionopohore, transporting ions through membranes and artificial lipid membranes and forming voltage-dependent ion channels in lipid bilayer membranes. Alamethicin is co-produced with a parallel, neutral, linear peptaibol complex (alamethicin F50). The relative composition of alamethicin is a variable mixture of the acidic and neutral complexes, but reports on alamethacin do not generally specify the ratio of F50 to F30, and comparative data between the complexes is scant.
General Description
Alamethicin belongs to the peptaibol family of antimicrobial peptides. It is mainly composed of hydrophobic amino acids. It possess a helical structure with a movable hinge region at Gly-11 position.
Purification Methods
Recrystallise alamethicin from MeOH. [Panday et al. J Am Chem Soc 99 8469 1977.] The acetate has m 195-180o from MeOH/Et2O, and the acetate-methyl ester [64936-53-4] has m 145-140o from aqueous MeOH.
ALAMETHICINRESEARCH GRADE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 16858 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
| Zhejiang Hangyu API Co., Ltd | +8617531972939 | China | 2944 | 58 | Inquiry |
| LEAPCHEM CO., LTD. | +86-852-30606658 | China | 43348 | 58 | Inquiry |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | China | 33350 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
| Wuhan Huangzhen Biochemical Co., Ltd | 13795480948 13795480948 | China | 4958 | 58 | Inquiry |






