Josamycin
- CAS No.
- 16846-24-5
- Chemical Name:
- Josamycin
- Synonyms
- C12662;EN 141;Jomybel;YL-704A3;Iosalide;Josacine;Josamina;Wilprafen;Vilprafen;JOSAMYCIN
- CBNumber:
- CB5444692
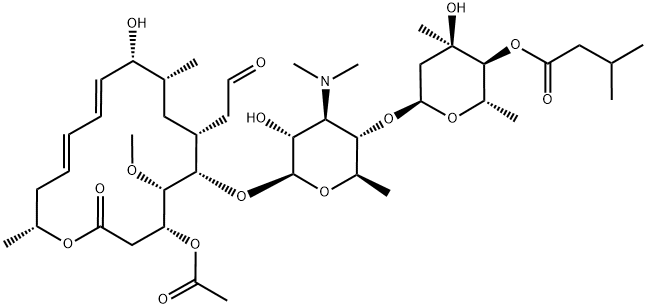
- Molecular Formula:
- C42H69NO15
- Molecular Weight:
- 827.99
- MOL File:
- 16846-24-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/4/10 11:19:08
| Melting point | 131.5℃ |
|---|---|
| alpha | D25 -70° (c = 1 in ethanol) |
| Boiling point | 763.27°C (rough estimate) |
| Density | 1.1547 (rough estimate) |
| refractive index | 1.6220 (estimate) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in ethanol |
| pka | 7.1 (40% aq methanol) |
| form | powder |
| color | white to slightly yellow |
| Merck | 13,5286 |
| Stability | Hygroscopic |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
| WGK Germany | 2 |
| RTECS | OH4725810 |
| F | 10 |
| Toxicity | LD50 oral in rat: > 7gm/kg |
Josamycin Chemical Properties,Uses,Production
Chemical Properties
Josamycin is a macrolide substance having antibacterial activity produced by the growth of Streptomyces narboensis var. josamyceticus.
Josamycin appears as white to yellowish white powder, slightly hygroscopic. Josamycin is very soluble in methanol or in ethanol, and very slightly soluble in water.
Uses
As a 16-membered ring macrolide antibiotic with antimicrobial activity against a wide range of pathogens. Josamycin can be particularly used in the treatment of Mycoplasma infection.
Josamycin is used to study the modification of phagocytosis and cytokine production by macrolide antibiotics, immunomodulatory effects, the suppression of matrix metalloproteinase production as well as study bacteria protein synthesis at the level of the the 23S rRNA, 50S ribosomal subunit.
Definition
ChEBI: A macrolide antibiotic produced by certain strains of Streptomyces narbonensis var. josamyceticus.
Pharmaceutical Applications
A naturally occurring antibiotic produced by Streptomyces narbonensis var. josamyceticus and belonging to the leucomycin group of macrolides. It is formulated for oral administration.
Many Gram-positive and Gram-negative anaerobes are susceptible, including Peptostreptococcus spp., Propionibacterium spp., Eubacterium spp. and Bacteroides spp.
After a single 1 g oral dose, a peak serum concentration of 2.74 mg/L was achieved 0.75 h after dosing. The AUC was 4.2 mg.h/L, and the apparent elimination half-life 1.5 h. Several inactive metabolites could be detected. It penetrates into saliva, tears and sweat, and achieves high levels in bile and lungs. It is mostly metabolized and excreted in the bile in an inactive form. Less than 20% of the dose appears in the urine, producing levels of around 50 mg/L.
The drug is generally well tolerated, producing only mild gastrointestinal disturbance. Its uses are similar to those of erythromycin. It is of limited availability.
Biological Activity
Josamycin inhibits bacterial protein biosynthesis by inhibiting peptidyltransferase and ribosomal translocation, and depleting the intracellular pools of aminoacyl-tRNAs available for protein synthesis by drop-off and incomplete peptidyl-tRNA hydrolase activity. It slows down formation of the first peptide bond of a nascent peptide in an amino acid-dependent way and inhibits formation of the second or third peptide bond.
Josamycin Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | China | 10326 | 58 | Inquiry |
| Hebei Yanxi Chemical Co., Ltd. | +86-17531190177; +8617531190177 | China | 5959 | 58 | Inquiry |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 | China | 436 | 58 | Inquiry |
| airuikechemical co., ltd. | +undefined86-15315557071 | China | 983 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21669 | 55 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9337 | 55 | Inquiry |
Related articles
- Josamycin: Pharmacodynamics, Pharmacokinetics and Indications
- Josamycin, a macrolide antibiotic, inhibits bacterial protein synthesis and is effective against various infections due to its....
- Apr 8,2024
- Josamycin:Uses,Pharmacodynamics,Toxicity,Preparation
- The Josamycin, with the CAS No: 16846-24-5, is also known as 3-Acetate 4B-(3-Methylbutanoate) leucoMycin V. This chemical’s mo....
- Dec 27,2022
16846-24-5(Josamycin)Related Search:
1of4
chevron_right




