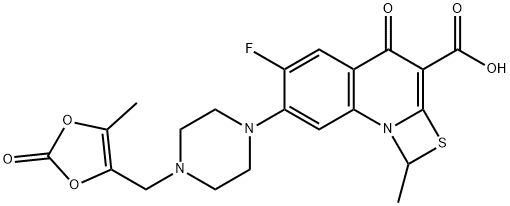
Prulifloxacin
- CAS No.
- 123447-62-1
- Chemical Name:
- Prulifloxacin
- Synonyms
- nm441;Sword;Pruvel;Quisnon;CS-1352;AF 3012;Plumfloxacin;PRULIFLOXACIN;Prulifloxacine;Prulioxacin-d8
- CBNumber:
- CB5474525
- Molecular Formula:
- C21H20FN3O6S
- Molecular Weight:
- 461.46
- MOL File:
- 123447-62-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/6/8 17:06:35
| Melting point | 211-214°C |
|---|---|
| Boiling point | 633.2±65.0 °C(Predicted) |
| Density | 1.62±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | 1 M NaOH: soluble25ML, clear, colorless (Solvent: 1 mg + 25 mL of NaOH) |
| pka | 5.85±0.40(Predicted) |
| form | White to yellow crystalline solid. |
| color | Off-White to Pale Yellow |
| λmax | 276nm(H2O)(lit.) |
| Merck | 14,7908 |
| CAS DataBase Reference | 123447-62-1(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| RTECS | XJ0600000 |
| HS Code | 2941.90.3000 |
Prulifloxacin price More Price(2)
Prulifloxacin Chemical Properties,Uses,Production
Description
Prulifloxacin was launched as the third fluoroquinone. It was introduced in Japan as an oral treatment for urinary tract infections (UTls), respiratory tract infections (RTls) and bacterial pneumoniae. It can be synthesized in 10 steps from commercially available 3,4-difluoroaniline. Key steps involve the cyclization of 6,7-difluoro-rl-hydroxy-2- thioquinoline-3carboxylic acid ethyl ester with 1 ,I-dibromomethane to give the corresponding thiazeto-[3,2a]quinoline. Aromatic nucleophilic substitution of the 7-fluoro atom with piperazine followed by hydrolysis of the ethyl ester and finally alkylation of the piperazinyl moiety with 4-(bromomethyl)-5-methyl-l ,bdioxol-Bone complete the synthesis. Prulifloxacin is a lipophilic prodrug, which is rapidly hydrolyzed to the corresponding Ndealkylated piperazine, NM 394, by paraoxonase type enzymes in blood and liver following intestinal absorption. The DNA gyrase inhibitor NM 394 accounts for all antimicrobial activity: it shows a similar or greater activity against gram-positive bacteria compared to ciprofloxacin, and a greater activity in the case of gram-negative bacteria. In clinical studies, prulifloxacin has shown good efficacy against UTls and RTls. The drug is mainly excreted in the urine and in the feces as unchanged NM 394, which has a plasma half-life of approximately 8 h. Phototoxicity in animal models is less severe than with other quinolones. Prulifloxacin is well tolerated with an adverse effect profile similar to that of other fluoroquinolones.
Chemical Properties
Off-White Solid
Uses
Prulifloxacin is a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class. Prulifloxacin is a prodrug for active metabolite, Ulifloxacin. Antibacterial.
Pharmaceutical Applications
A lipophilic prodrug which is very rapidly metabolized by esterase into ulifloxacin, a 6-fluoro, 7-piperazinyl thiazetoquinoline.
Ulifloxacin is moderately active against Staph. aureus (MIC 0.4–0.8 mg/L) and inactive against Str. pneumoniae (MIC 2–8 mg/L) as well as against Enterococcus spp. Against Enterobacteriaceae (MIC 0.05–0.8 mg/L) and Ps. aeruginosa (MIC 0.2–0.8 mg/L) activity is similar to that of ciprofloxacin. It is active against fastidious Gram-negative bacilli, but not against anaerobes and non-fermentative Gram-negative bacilli. Activity against Acinetobacter spp. is modest.
Prulifloxacin is rapidly converted into ulifloxacin and after 3 h is no longer detected in blood. In volunteers receiving a single oral dose, peak plasma concentrations of 0.68 mg/L (300 mg dose) to 1.88 mg/L (for 400 mg dose) were attained between 0.67 and 1.25 h. The mean apparent elimination half-life was 8 h and the mean cumulative elimination rate in urine within 48 h was 31–46%. Other inactive metabolites account for 7% of the dose. Half the administered dose is eliminated in feces within 72 h as ulifloxacin and 4% as prulifloxacin. Protein binding is 45%.
Prulifloxacin Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Honour Lab Limited | +919845977466 | Telangana, India | 164 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Aspen Biopharma Labs Pvt Ltd | +91-9248058660 +91-9248058662 | Telangana, India | 234 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Sai Prathyu Marketing | 91-9666672409 | Hyderabad, India | 162 | 58 | Inquiry |
| Festiva Pharma | 08048372775Ext 728 | Gujarat, India | 238 | 58 | Inquiry |
123447-62-1(Prulifloxacin)Related Search:
1of4
chevron_right




