Moxifloxacin
- CAS No.
- 151096-09-2
- Chemical Name:
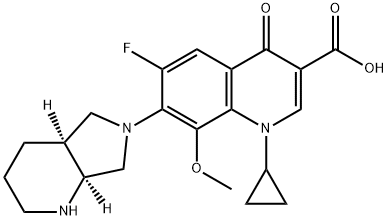
- Moxifloxacin
- Synonyms
- 1-Cyclopropyl-6-fluoro-8-methoxy-7-(octahydro-pyrrolo[3,4-b]pyridin-6-yl)-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid;7-[(4aS,7aS)-octahydro-1H-pyrrolo[3,4-b]pyridin-6-yl]-1-cyclopropyl-6-fluoro-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid;Avdox;CS-83;Aids070017;Aids-070017;MOXIFLOXACIN;MAXIOFFOXACIN;Moxifloxacin(Hclform);Moxifloxacin USP/EP/BP
- CBNumber:
- CB0191432
- Molecular Formula:
- C21H24FN3O4
- Molecular Weight:
- 401.43
- MOL File:
- 151096-09-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/21 22:41:43
| Melting point | 203-208 C |
|---|---|
| alpha | 23D -193° |
| Boiling point | 636.4±55.0 °C(Predicted) |
| Density | 1.408±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C(protect from light) |
| solubility | Acetonitrile (Slightly), DMSO (Slightly, Heated, Sonicated), Methanol (Slightly) |
| pka | 6.43±0.50(Predicted) |
| form | Solid |
| color | Off-White to Light Yellow |
| Stability | Hygroscopic |
| CAS DataBase Reference | 151096-09-2(CAS DataBase Reference) |
| EPA Substance Registry System | 3-Quinolinecarboxylic acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo- (151096-09-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H302-H412 |
| Precautionary statements | P273-P501-P264-P280-P305+P351+P338-P337+P313P-P264-P270-P301+P312-P330-P501 |
| Hazard Codes | Xn |
| Risk Statements | 22-40 |
| Safety Statements | 36/37 |
Moxifloxacin Chemical Properties,Uses,Production
Uses
Moxifloxacin is an antibiotic for the treatment of bacterial infections like bacterial sinusitis, acute bacterial exacerbations of chronic bronchitis, and community-acquired pneumonia.
Definition
ChEBI: A quinolone that consits of 4-oxo-1,4-dihydroquinoline-3-carboxylic acid bearing a cyclopropyl substituent at position 1, a fluoro substitiuent at position 6, a (4aS,7aS)-octahydro-6H-pyrrolo[3,4-b /ital>]pyridin-6-yl group at position 7 and a methoxy substituent at position 8. A member of the fluoroquinolone class of antibacterial agents.
Antimicrobial activity
It displays good activity in vitro against Enterobacteriaceae and fastidious Gram-negative bacilli such as H. influenzae and Mor. catarrhalis, as well as against Grampositive cocci including Str. pneumoniae, but is poorly active against Enterococcus spp. Activity against non-fermentative Gramnegative bacilli is species dependent: Acinetobacter spp. (MIC 0.006–2.0 mg/L) and Sten. maltophilia (MIC 0.5–2.0 mg/L) are partially susceptible in vitro, but it has poor activity against Ps. aeruginosa and other non-fermenting Gram-negative rods. It displays good in-vitro activity against Ch. pneumoniae, C. trachomatis, mycoplasmas (including M. pneumoniae), L. pneumophila and B. fragilis. Although highly active against M. tuberculosis, it is less active against the M. avium complex, M. intracellulare, M. chelonei and M. fortuitum.
Pharmaceutical Applications
fluoroquinolone substituted with an 8-methoxy group and a 7-diazabicyclononyl moiety, formulated as the hydrochloride for oral or intravenous use.
Pharmacokinetics
absorption and distribution
By the oral route, drug uptake is rapid, with moderate variability. As with all quinolones iron and antacids significantly reduce the bioavailability. No significant drug interactions with theophylline, itraconazole, probenecid or oral contraceptives have been found. In escalating dose studies (50–80 mg doses), Cmax and AUC values increased proportionally to the dose.
It is widely distributed throughout the body and into many tissues in concentrations exceeding those in plasma. Around 50–80% of plasma concentrations penetrate into CSF if the meninges are inflamed. The apparent plasma half-life is 15.6 h.
Metabolism and excretion
Biliary elimination and metabolism are the main elimination pathways. About 19.3% of the administered dose is eliminated in the urine, with a bioavailability of 86.2%. Urinary excretion is via glomerular filtration and tubular reabsorption. Two main metabolites are recovered: M1 (a sulfocompound) and M2 (a glucuronide). M1 is mainly eliminated in feces (34.4%) and only 2.5% in urine: M2 is eliminated in urine (13.8%).
Clinical Use
Acute bacterial exacerbations of chronic bronchitis and community
acquired pneumonia
Acute bacterial sinusitis
Treatment of complicated skin and soft-tissue infections caused by methicillin-susceptible Staph. aureus and Gram-negative rods (i.v. formulation)
Treatment of complicated intra-abdominal infections (i.v. formulation)
Side effects
Adverse events are similar to those for other fluoroquinolones. Phototoxicity rates are not significantly above placebo levels. Gastrointestinal side effects are the most common, particularly nausea, diarrhea, abdominal pain and vomiting. Dizziness and headache may occur as well as allergic reactions. Attention has been drawn to a potential to cause lifethreatening hepatotoxicity. Moxifloxacin has the potential to prolong the QTc interval in some patients but the clinical significance of this phenomenon is unclear.
Moxifloxacin Preparation Products And Raw materials
Raw materials
1of5
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANWITA APIS | +919000311012 | Hyderabad, India | 198 | 58 | Inquiry |
| J S LABS | +91-7330612784 +91-7330612784 | Tamil Nadu, India | 160 | 58 | Inquiry |
| Macleods Pharmaceuticals Limited | +91-2266762800 +91-2266762800 | Maharashtra, India | 116 | 58 | Inquiry |
| HRV Global Life Sciences | +91-9820219686 +91-9820219686 | Telangana, India | 379 | 58 | Inquiry |
| Vanquest Pharma Pvt Ltd | +91-9920897640 +91-9920897640 | Mumbai, India | 36 | 58 | Inquiry |
| Cureworth Drugs And Intermediates Pvt Ltd | +91-7738155701 +91-7686877773 | Maharashtra, India | 4 | 58 | Inquiry |
| Aarti Drugs Ltd (part of Aarti Group of Industries) | +91-2224019025 +91-9136988221 | Maharashtra, India | 57 | 58 | Inquiry |
| Rini Life Science Pvt Ltd | +91-7314202701 +91-7314202701 | Madhya Pradesh, India | 12 | 58 | Inquiry |
| Alfa Omega Pharma | +91-8050045945 +91-9972665399 | Maharashtra, India | 126 | 58 | Inquiry |
| Niksan Pharmaceutical | +91-9537871777 +91-9537871777 | Gujarat, India | 39 | 58 | Inquiry |
Related articles
- Toxicity of Moxifloxacin
- Moxifloxacin (Bay 12-8039) is a fourth-generation synthetic fluoroquinolone developed by Bayer Pharmaceuticals in the early 19....
- Mar 15,2022
151096-09-2(Moxifloxacin)Related Search:
1of4
chevron_right




