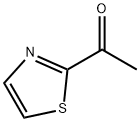
2-Acetylthiazole
- CAS No.
- 24295-03-2
- Chemical Name:
- 2-Acetylthiazole
- Synonyms
- 2-Acetylthiazol;1-(thiazol-2-yl)ethan-1-one;1-(1,3-Thiazol-2-yl)ethanone;FEMA 3328;Acetylthiazole;2-ACETYLTHIAZOLE;FEMA NUMBER 3328;2-acetylthiazone;THIAZOLE-2-ACETYL;2-Acetylo thiazole
- CBNumber:
- CB6235983
- Molecular Formula:
- C5H5NOS
- Molecular Weight:
- 127.16
- MOL File:
- 24295-03-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/16 16:27:54
| Melting point | 65.5°C |
|---|---|
| Boiling point | 89-91 °C/12 mmHg (lit.) |
| Density | 1.227 g/mL at 25 °C (lit.) |
| FEMA | 3328 | 2-ACETYLTHIAZOLE |
| refractive index |
n |
| Flash point | 173 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| pka | 0.05±0.10(Predicted) |
| form | Powder or Crystals |
| Specific Gravity | 1.23 |
| color | White to slightly yellow |
| Odor | at 0.10 % in dipropylene glycol. nutty popcorn roasted peanuts hazelnut |
| Odor Type | popcorn |
| Sensitive | Stench |
| JECFA Number | 1041 |
| BRN | 109803 |
| LogP | 0.37 |
| CAS DataBase Reference | 24295-03-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Acetylthiazole(24295-03-2) |
| EPA Substance Registry System | Ethanone, 1-(2-thiazolyl)- (24295-03-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H227-H302-H317-H319 | |||||||||
| Precautionary statements | P280-P305+P351+P338-P210-P264-P270-P301+P312+P330-P305+P351+P338+P337+P313-P403+P235-P501-P280f | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-36-43-36/37/38 | |||||||||
| Safety Statements | 26-36/37-24/25-36 | |||||||||
| RIDADR | 3334 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 13 | |||||||||
| Hazard Note | Irritant/Stench | |||||||||
| TSCA | T | |||||||||
| HazardClass | STENCH | |||||||||
| HS Code | 29341000 | |||||||||
| NFPA 704 |
|
2-Acetylthiazole price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | W332801 | 2-Acetylthiazole ≥99%, FG | 24295-03-2 | 1SAMPLE-K | ₹5141.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W332801 | 2-Acetylthiazole ≥99%, FG | 24295-03-2 | 100G | ₹27214.05 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W332801 | 2-Acetylthiazole ≥99%, FG | 24295-03-2 | 1KG | ₹102999.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W332801 | 2-Acetylthiazole ≥99%, FG | 24295-03-2 | 5KG | ₹470671 | 2022-06-14 | Buy |
| Sigma-Aldrich | 288411 | 2-Acetylthiazole 99% | 24295-03-2 | 1G | ₹3518.13 | 2022-06-14 | Buy |
2-Acetylthiazole Chemical Properties,Uses,Production
Chemical Properties
2-Acetylthiazole is a colorless to pale yellow liquid with green onion, herbal, grassy, hazelnuts, roasted meat, caramel, corn, and butter odor. Natural products are found in beef broth. Slightly soluble with water, soluble in most organic solvents. It is used in foods as antioxidant, as appearance control agent, and as flavoring ingredient.
Occurrence
2-Acetylthiazole is naturally found in raw asparagus, cooked asparagus, kohlrabi, cooked or boiled potatoes, turkey (roasted), raw chicken, boiled and cooked beef, grilled and roasted beef, pork liver, beer, Finnish whiskey, heated beans, other varieties of mushroom, rice bran, maize, and other natural sources.
Uses
2-Acetylthiazole can be used as a flavoring agent in food industries. It may be used as a food additive in the preparation of ′fragrant′ rice. It also was used in the preparation of triazolothiazoles, chiral alcohols and in aldol condensation reactions.
Preparation
2-Acetylthiazole is formed by oxidation of the corresponding carbinol using dichromate.
General Description
2-Acetylthiazole is a volatile flavoring substance generally formed by the Maillard reaction between an amino acid and carbonyl compounds. It is reported to occur in canned sweet corn products, cooked pine mushroom, cooked asparagus and roasted beef.
Purification Methods
Check NMR spectrum; if it is not too bad, distil it through an efficient column in a vacuum. The oxime sublimes at 140-145o, m 159o, and when crystallised from H2O has m 163-165.5o. [Erlenmeyer et al. Helv Chim Acta 31 1142 1948, Waisvisz et al. J Am Chem Soc 79 4524 1957, Menasseé et al. Helv Chim Acta 40 554 1957, Beilstein 27 IV 2617.]
2-Acetylthiazole Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Hebei Yanxi Chemical Co., Ltd. | +86-17531190177; +8617531190177 | China | 5873 | 58 | Inquiry |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | China | 20294 | 58 | Inquiry |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | China | 3002 | 58 | Inquiry |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 | China | 646 | 58 | Inquiry |
| JINING XINHE CHEMICAL CO., LTD | +8615318402391 | China | 808 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 18648 | 58 | Inquiry |





