Tigecycline
- CAS No.
- 220620-09-7
- Chemical Name:
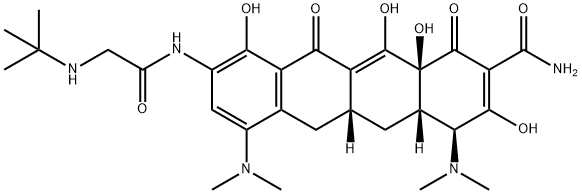
- Tigecycline
- Synonyms
- Tygacil;(4s,4as,5ar,12as)-4,7-bis(dimethylamino)-9-[(tert-butylamino)acetamido]-3,10,12,12a-tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracen-2-carboxamide;Tig;ecycL;CS-131;TigecycL;igecycline;Tegecycline;tigecycline;WAY-GAR 936
- CBNumber:
- CB6248215
- Molecular Formula:
- C29H39N5O8
- Molecular Weight:
- 585.65
- MOL File:
- 220620-09-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/8 15:48:26
| Melting point | 164-166°C |
|---|---|
| Boiling point | 890.9±65.0 °C(Predicted) |
| Density | 1.45±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in DMSO (up to at least 25 mg/ml). |
| pka | 4.50±1.00(Predicted) |
| form | Orange powder |
| color | Orange |
| Merck | 14,9432 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
| InChIKey | FPZLLRFZJZRHSY-HJYUBDRYSA-N |
| SMILES | C1(=O)[C@]2(O)[C@@]([H])(C[C@@]3([H])C(=C2O)C(=O)C2=C(C(N(C)C)=CC(NC(CNC(C)(C)C)=O)=C2O)C3)[C@H](N(C)C)C(O)=C1C(N)=O |
| CAS DataBase Reference | 220620-09-7(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H319-H360D-H372-H410 | |||||||||
| Precautionary statements | P202-P260-P264-P273-P305+P351+P338-P308+P313 | |||||||||
| Safety Statements | 24/25 | |||||||||
| RIDADR | 3077 | |||||||||
| RTECS | QI7619500 | |||||||||
| HazardClass | 9 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29419090 | |||||||||
| NFPA 704 |
|
Tigecycline price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | PHR2591 | Tigecycline certified reference material, pharmaceutical secondary standard | 220620-09-7 | 500MG | ₹22342.8 | 2022-06-14 | Buy |
| TCI Chemicals (India) | T3589 | Tigecycline | 220620-09-7 | 100MG | ₹7200 | 2022-05-26 | Buy |
| TCI Chemicals (India) | T3589 | Tigecycline | 220620-09-7 | 500MG | ₹14600 | 2022-05-26 | Buy |
Tigecycline Chemical Properties,Uses,Production
Description
The emergence of drug-resistant bacteria has diminished the clinical utility of the
tetracyclines. Research to circumvent the efflux and ribosomal protection mechanisms
of bacteria has led to the development of the glycylcyclines. Tigecycline is
the first glycylcycline antibiotic to launch for the parenteral treatment of
baterial infection, including complicated intra-abdominal and skin infections. Its mechanism of action involves inhibiting protein translation in bacteria by binding
to the 30S ribosomal subunit and blocking entry of amino-acyl tRNA molecules
into the A site of the ribosome to effectively prevent incorporation of amino acid
residues into elongating peptide chains. Presumably, ribosomal protection proteins
are ineffective against tigecycline due to its higher affinity for ribosomal binding
compared to tetracyclines (approximately 16-fold). In addition, tigecycline may be
resistant to efflux mechanisms by either their inability to translocate it across the
cytoplasmic membrane due to steric complications or simply by their failure to
recognize the molecule.
Chemical Properties
Orange Solid
Uses
Tigecycline is a semi-synthetic tetracycline prepared by the introduction of a tert-butylaminoacetamido group into a previously unexplored and un-substituted region of existing tetracyclines. Like other tetracyclines, tigecycline acts by reversibly binding to the 30S ribosomal subunit and inhibits protein translation by blocking entry of aminoacyl-tRNA into the ribosome A site. The enhanced activity can be attributed to stronger binding affinity, thus minimising the impact of existing mechanisms of resistance. Tigecycline is regarded as the first of a new class of glycylcyline antibiotics. Critical comparison to the tetracycline class appears to be lacking in the literature.
Definition
ChEBI: Tetracycline in which the hydroxy group at position 5 and the methyl group at position 6 are replaced by hydrogen, and with a dimethylamino substituent and an (N-tert-butylglycyl)amino substituent at positions 7 and 9, respe tively. A glycylcycline antibiotic, it has activity against a broad range of Gram-positive and Gram-negative bacteria, including tetracycline-resistant organisms. It is used for the intravenous treatment of complicated skin and skin structure infections ca sed by susceptible organisms.
Antimicrobial activity
It is as potent as, or more potent than,
earlier tetracyclines and activity is retained against strains
expressing acquired tetracycline resistance determinants. It
displays better activity than tetracycline, doxycycline or
minocycline against Streptococcus spp. and against Enterococcus
faecalis and E. faecium. Among Gram-negative organisms it
displays improved activity against Citrobacter freundii,
Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae,
Salmonella spp., Serratia marcescens and Shigella spp. The
spectrum includes rapidly growing mycobacteria. Ps. aeruginosa,
Pr. mirabilis, other Proteus spp. and some strains of
Corynebacterium jeikeium are resistant. Activity against strains
expressing acquired resistance to earlier tetracyclines is
attributed to failure of the MFS efflux pumps to recognize
tigecycline, and to a novel mechanism of ribosome binding
that permits tigecycline to overcome ribosomal protection
mechanisms.
Comparative susceptibility data for some atypical pathogens
are not available. However, in common with earlier
tetracyclines,
it is active against Chlamydophila and Mycoplasma
spp. and rapidly growing Mycobacteria spp. It is less active
than minocycline or tetracycline against U. urealyticum.
General Description
Tigecycline (Tygacil) is a first-in-class (a glycylcycline) intravenousantibiotic that was designed to circumvent manyimportant bacterial resistance mechanisms. It is not affectedby resistance mechanisms such as ribosomal protection, effluxpumps, target site modifications, β-lactamases, or DNAgyrase mutations. Tigecycline binds to the 30S ribosomalsubunit and blocks peptide synthesis. The glycylcyclinesbind to the ribosome with five times the affinity of commontetracyclines. Tigecycline also possesses a novel mechanismof action, interfering with the mechanism of ribosomal protectionproteins. Tigecycline, unlike common tetracyclines,is not expelled from the bacterial cell by efflux pumpingprocesses.
Tigecycline is recommended for the treatment of complicatedskin and skin structure infections caused by E. coli,E. faecalis (vancomycin-susceptible isolates), S. aureus(methicillin-susceptible and methicillin-resistant isolates),S. pyogenes, and B. fragilis among others. Tigecycline is alsoindicated for complicated intra-abdominal infections causedby strains of Clostridium, Enterobacter, Klebsiella, andBacteroides. To reduce the development of resistance to tigecycline,it is recommended that this antibiotic be used onlyfor those infections caused by proven susceptible bacteria.Glycylcyclines are structurally similar to tetracyclines,and appear to have similar adverse effects. These mayinclude photosensitivity, pancreatitis, and pseudotumorcerebri. Nausea and vomiting have been reported.
Pharmaceutical Applications
9-T-butylglycylamido-minocycline. A compound of the glycylcycline class available as a powder for intravenous infusion.
Pharmacokinetics
Cmax 100 mg intravenous infusion (1 h): 0.85–1 mg/L
Plasma half-life: 37–67 h
Volume of distribution: 7–10 L/kg
Plasma protein binding: 68%
Distribution and excretion
It is widely distributed and is concentrated in the gallbladder,
colon and lung. The volume of distribution is dose related and
variable, but is generally greater than that of older tetracyclines.
CSF penetration is poor. Tigecycline is excreted in the
feces and urine predominantly as the unchanged molecule.
The elimination half-life is long (37–67 h). Tigecycline clearance
is decreased by 20% in patients with renal failure. No
dosage adjustments are apparently necessary for tigecycline
in patients with renal impairment.
Clinical Use
Complicated skin and skin structure infections
Complicated intra-abdominal infections
Community-acquired bacterial pneumonia
Recommended principally for the treatment of infections with
multiresistant organisms.
Side effects
Side effects typical of the group, including nausea, vomiting, diarrhea and headache, occur. Occasional cases of pancreatitis, hypoproteinemia, antibiotic-associated colitis and thrombocytopenia have also been reported.
Tigecycline Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| THE MOLECULEZ | +91-7506703683 +91-7506703683 | Maharashtra, India | 79 | 58 | Inquiry |
| Micro Orgo Chem | +91-91-022-24969510 +91-9082984052 | Mumbai, India | 66 | 58 | Inquiry |
| Lewens Labs | +917043057100 | Gujarat, India | 30 | 58 | Inquiry |
| Prayosha Health Care Pvt Ltd | +91-9924448881 +91-9924448881 | Gujarat, India | 15 | 58 | Inquiry |
| MELODY HEALTHCARE PVT LTD | +undefined2228780912 | Maharashtra, India | 20 | 58 | Inquiry |
| Salvavidas Pharmaceutical Pvt., Ltd. | +91-2612538898 +91-9327127459 | Gujarat, India | 34 | 58 | Inquiry |
| Basil Drugs AND Pharmaceuticals Pvt Ltd | +91-2249700250 +91-9619320820 | Mumbai, India | 108 | 58 | Inquiry |
| Moleculochem | 9811853366 | New Delhi, India | 25 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Jigs Chemical ltd | 58 |
| THE MOLECULEZ | 58 |
| Micro Orgo Chem | 58 |
| Lewens Labs | 58 |
| Prayosha Health Care Pvt Ltd | 58 |
| MELODY HEALTHCARE PVT LTD | 58 |
| Salvavidas Pharmaceutical Pvt., Ltd. | 58 |
| Basil Drugs AND Pharmaceuticals Pvt Ltd | 58 |
| Moleculochem | 58 |
| Ralington Pharma | 58 |
Related articles
- The first glycylcycline---Tigecycline
- Tigecycline is produced by Wyeth under the trade name of Tygacils. The modification of the tetracycline nucleus led to a reduc....
- Mar 18,2022
220620-09-7(Tigecycline)Related Search:
1of4
chevron_right




