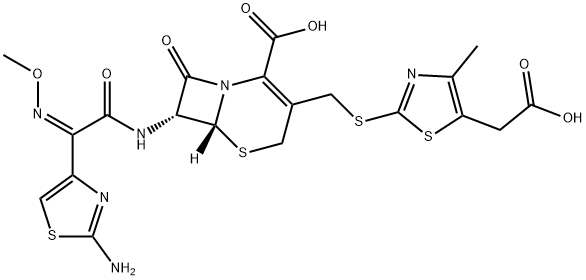
Cefodizime
- CAS No.
- 69739-16-8
- Chemical Name:
- Cefodizime
- Synonyms
- FIR-221;Estrofan;Cefodizim;Cefodizme;CEFODIZIME;Cefodizime,CDZM;CefodizMe SodiuM;Sodium cefodizime;CEFODIZIME FREE ACID;Cefodizime USP/EP/BP
- CBNumber:
- CB6299020
- Molecular Formula:
- C20H20N6O7S4
- Molecular Weight:
- 584.67
- MOL File:
- 69739-16-8.mol
- Modify Date:
- 2024/4/28 15:15:04
| Melting point | >170°C (dec.) |
|---|---|
| alpha | D25 -55.9° |
| Density | 1.86±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated) |
| form | Solid |
| pka | pK1 2.85; pK2 3.37; pK3 4.18(at 25℃) |
| color | White to Off-White |
| CAS DataBase Reference | 69739-16-8(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Hazard Codes | Xi |
|---|---|
| Risk Statements | 36/37/38-42/43 |
| Safety Statements | 22-26-36/37/39 |
Cefodizime Chemical Properties,Uses,Production
Chemical Properties
Cefodizime Sodium is a white to light yellowish white crystalline powder. It has no odor or a slightly peculiar odor and has a bitter taste. It is highly soluble in water (around 270g/L) but only sparingly soluble in ethanol or ether.
Definition
ChEBI: Cefodizime is a cephalosporin compound having 5-(carboxymethyl)-4-methyl-1,3-thiazol-2-yl]sulfanyl}methyl and [2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino side groups located at positions 3 and 7 respectively. It has a role as an antibacterial drug and an EC 1.14.18.1 (tyrosinase) inhibitor. It is a cephalosporin, a member of 1,3-thiazoles and an oxime O-ether.
Preparation
The synthesis of cefodizime involved the following steps:
Dissolve 6.1g of (2-mercapto-4-methylthiazol-5-yl) acetic acid in water and adjust the pH to 6.5 by adding 2 mol/L sodium hydroxide.
Heat the solution to 70°C and then add a solution of 12g of cefotaxime in 75ml of water with stirring.
Keep stirring the mixture at 70°C for 3 hours while maintaining the pH at 6.5 by adding 2 mol/L sodium hydroxide.
Cool the mixture to room temperature and acidify it to pH 2.8.
Filter the resulting precipitate and wash it with water.
Dry the precipitate under vacuum and in the presence of phosphorus pentoxide.
As a result, 10g of cefodizime is obtained.
Cefodizime, an aminothiazolylcephalosporin. V. Synthesis and structure-activity relationships in the cefodizime series. DOI: 10.7164/antibiotics.40.29
Cefodizime Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | China | 12456 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | China | 8826 | 58 | Inquiry |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | China | 6387 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| SIMAGCHEM CORP | +86-13806087780 | China | 17367 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
69739-16-8(Cefodizime)Related Search:
1of4
chevron_right




