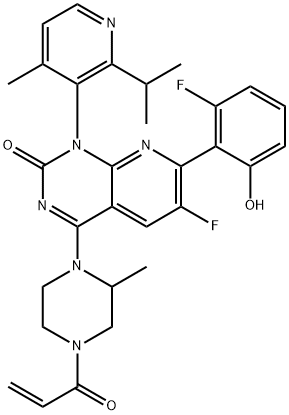
Sotorasib
- CAS No.
- 2296729-00-3
- Chemical Name:
- Sotorasib
- Synonyms
- Sotorasib;AMG-510(Sotorasib);Storasib;Sotoracib;rac AMG-510;CID 137278711;2H7]-Sotorasib;Sotolabu (AMG-510);Sotorasib AMG510 AMG-510;Sotorasib, 10 mM in DMSO
- CBNumber:
- CB65475731
- Molecular Formula:
- C30H30F2N6O3
- Molecular Weight:
- 560.61
- MOL File:
- 2296729-00-3.mol
- Modify Date:
- 2025/4/28 21:36:36
| Boiling point | 730.5±70.0 °C(Predicted) |
|---|---|
| Density | 1.36±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C, stored under nitrogen |
| solubility | DMSO : 100 mg/mL (178.38 mM; Need ultrasonic)| |
| pka | 6.52±0.35(Predicted) |
| form | Solid |
| color | White to yellow |
| Water Solubility | Water : 33.33 mg/mL (59.46 mM; ultrasonic and adjust pH to 11 with NaOH) |
| InChIKey | NXQKSXLFSAEQCZ-UHFFFAOYSA-N |
| SMILES | O=C1N=C(N2CCN(C(=O)C=C)CC2C)C2=CC(F)=C(C3C(=CC=CC=3F)O)N=C2N1C1C(=CC=NC=1C(C)C)C |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
Sotorasib Chemical Properties,Uses,Production
brand name
Lumakras
General Description
Sotorasib, also known as AMG-510, is an acrylamide derived KRAS inhibitor developed by Amgen. It is indicated in the treatment of adult patients with KRAS G12C mutant non small cell lung cancer. This mutation makes up >50% of all KRAS mutations. Mutant KRAS discovered in 1982 but was not considered a druggable target until the mid-2010s. It is the first experimental KRAS inhibitor. The drug [MRTX849] is also currently being developed and has the same target. Sotorasib was granted FDA approval on 28 May 2021.
General Description
Class: non-kinase; Treatment: KRAS(G12C) NSCLC; Other name: AMG 510; Elimination half-life: 5.5 h; Protein binding = 89%
Side effects
Sotorasib may cause breathing problems that could lead to death. Get emergency medical help if you have new or worsening fever, cough, or shortness of breath.
Common side effects may include:
nausea, diarrhea;
cough;
liver problems;
pain in your bones, joints, or muscles;
tiredness;
abnormal lab tests.
For 28 patients (22%), side effects led to a pause, a reduction, or both, in the dose of sotorasib. Nine people (7%) stopped the treatment because of side effects.
www.everydayhealth.com/drugs/sotorasib
target
Primary target: KRAS(G12C)
Mode of action
Sotorasib is an orally available inhibitor of the specific KRAS mutation, p.G12C, with potential antineoplastic activity. Upon oral administration, sotorasib selectively targets, binds to and inhibits the activity of the KRAS p.G12C mutant. This may inhibit growth in KRAS p.G12C-expressing tumor cells. The KRAS p.G12C mutation is seen in some tumor cell types and plays a key role in tumor cell proliferation.
Sotorasib Preparation Products And Raw materials
Raw materials
Preparation Products
Sotorasib Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Bulat Pharmaceutical Pvt Ltd | +91-8448085659 +91-8448085660 | Haryana, India | 123 | 58 | Inquiry |
| Aspen Biopharma Labs Pvt Ltd | +91-9248058660 +91-9248058662 | Telangana, India | 234 | 58 | Inquiry |
| ENBRIDGE PHARMTECH CO., LTD. | +8613812269233 | China | 322 | 58 | Inquiry |
| Heading (Nanjing)Pharmtechnologies Co., Ltd. | +86-25-58467899-832 +86-13382406280 | China | 46 | 58 | Inquiry |
| Tianjin Kilo Pharmaceutical Sci-Tech Co., Ltd | +86-02223869539 +86-15560057295 | China | 27 | 58 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 33024 | 60 | Inquiry |
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 | China | 8497 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49732 | 58 | Inquiry |
| Chengdu Aslee Biopharmaceuticals, Inc. | 28-85305008 | CHINA | 964 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 32222 | 58 | Inquiry |
Related articles
- How is Sotorasib synthesised?
- The synthetic route of Sotorasib is completed in three steps, starting with the amidation of nicotinic acid derivatives to pro....
- Jan 26,2024





