NICKEL CYANIDE
- CAS No.
- 557-19-7
- Chemical Name:
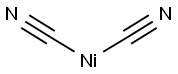
- NICKEL CYANIDE
- Synonyms
- dicyanonickel;NICKEL CYANIDE;nickeldicyanide;nickel(ii)cyanide;Dicyanonickel(II);rcrawastenumberp074;Nickel(II)dicyanide;nickelcyanide(ni(cn)2);NICKEL CYANIDE TETRAHYDRATE;NICKEL CYANIDE ISO 9001:2015 REACH
- CBNumber:
- CB6739113
- Molecular Formula:
- C2N2Ni
- Molecular Weight:
- 110.73
- MOL File:
- 557-19-7.mol
- Modify Date:
- 2023/5/4 15:14:32
| Melting point | 200°C -4H₂O |
|---|---|
| Density | 2.393 g/cm3 |
| form | solid |
| Merck | 14,6506 |
| Stability | Stable, but reacts violently with magnesium. |
| EPA Substance Registry System | Nickel(II) cyanide (557-19-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H317-H334-H350i-H372-H410 |
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P261-P285-P304+P341-P342+P311-P501-P260-P264-P270-P314-P501-P273-P391-P501 |
| RIDADR | UN1653 |
| HazardClass | 6.1 |
| PackingGroup | II |
| HS Code | 28371990 |
NICKEL CYANIDE Chemical Properties,Uses,Production
Chemical Properties
Nickel cyanide is a yellowish-brown plates or powder that may change to a green color by absorbing moisture. Odor is a weak almond odor like cyanide.
Physical properties
The tetrahydrate, Ni(CN)2?4H2O constitutes apple green plates or powder; loses water of crystallization on heating at 200°C; decomposes on further heating; insoluble in water; slightly soluble in dilute acids; soluble in potassium cyanide solution and in ammonia, caustic soda, caustic potash and other bases.
Uses
Metallurgy, electroplating.
Preparation
Nickel cyanide is prepared by treating a soluble nickel salt, such as nickel chloride or nickel sulfate, with potassium cyanide solution: Ni2+ + 2CNˉ → Ni(CN)2
The product is a tetrahydrate, Ni(CN)2?4H2O, which on heating at 200°C yields yellow-brown anhydrous salt, Ni(CN)2.
Reactions
The most interesting reaction of nickel(II) cyanide is the formation of clathrate compounds in the presence of ammonia. When a solution of Ni(CN)2 in aqueous ammonia is shaken with benzene, a pale violet precipitate of the benzene clathrate Ni(CN)2?NH3?C6H6 is obtained. In this compound the Ni and CN groups form layers with ammonia molecules bonded above and below the planes of the layers on alternate nickel atoms. Thus half the nickel atoms are octahedrally surrounded by nitrogen (Ni-N=2.15 ?, N1-NH3 = 2.06 à) and half are planar 4-coordinated by carbon (Ni-C=1.76?). The benzene molecules occupy the holes (cages) formed between the layers. The average magnetic moment per nickel atom is 2.2 BM, and it has thus been established that the 4- coordinate nickel atoms are diamagnetic while the 6-coordinate atoms are paramagnetic with two unpaired electron spins.
General Description
NICKEL CYANIDE is an apple-green powder or a green crystalline solid. Insoluble in water. Toxic by inhalation and by ingestion. Carcinogenic. Produces toxic oxides of nitrogen in fires.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
NICKEL CYANIDE is thermally unstable and easily oxidized. Weakly basic. Liberates flammable and lethally poisonous hydrogen cyanide gas on contact with acids or acid fumes. Undergoes violent reactions with fluorine, hypochlorites, nitric acid, nitrates, nitrites. Undergoes an explosive reaction if melted with nitrites or chlorates (at about 450°C). Emits highly toxic cyanide fumes when heated to decomposition. [Lewis, 3rd ed., 1993, p. 360, 912]. Undergoes an incandescent reaction if heated with magnesium [Mellor, 1940, vol. 4, p. 271].
Hazard
Highly toxic.
Health Hazard
DUST: POISONOUS IF INHALED. Irritating to eyes, nose and throat. SOLID: POISONOUS IF SWALLOWED. Irritating to skin and eyes.
Fire Hazard
Not flammable.
Safety Profile
Confirmed human carcinogen. A poison. Incandescent reaction when heated with magnesium. When heated to decomposition it emits very toxic fumes of CN-. See also CYANIDE and NICI(EL COMPOUNDS.
Potential Exposure
Nickel cyanide is used in metallurgy, electroplating and making other chemicals.
Shipping
UN1653 Nickel cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. and equipped in OSHA 1910.156. The only respirators recommended for fire fighting are SCBAs that have full facepieces and are operated in a pressure-demand or other positive-pressure mode.
Incompatibilities
Nnickel cyanide is thermally unstable and easily oxidized. Weakly basic. Liberates flammable and lethally poisonous hydrogen cyanide gas on contact with acids or acid fumes. Undergoes violent reactions with fluorine, hypochlorites, nitric acid, nitrates, nitrites. Undergoes an explosive reaction if melted with nitrites or chlorates (at about 450 C). Violent reaction with magnesium. Keep away from acids, active metals, heat, or CO2; contact can cause release of toxic cyanide gas.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
NICKEL CYANIDE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| City Chemical LLC | 2039322489 | United States | 168 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9003 | 58 | Inquiry |
| City Chemicals Corporation | 800-248-2436 | United States | 6462 | 72 | Inquiry |
| Aithaca Chemical Corp. | (516) 229-2330 | United States | 1684 | 30 | Inquiry |
| Pfaltz & Bauer, Inc. | (800) 225-5172 | United States | 6830 | 72 | Inquiry |
| VWR International | 800 932 5000 | United Kingdom | 6554 | 82 | Inquiry |
| MP Biomedicals, Inc. | 949 833 2500 | United States | 6566 | 81 | Inquiry |
| Service Chemical Inc. | 888-895-6920 | Germany | 6373 | 71 | Inquiry |
| 3B Scientific Corporation | 847.281.9822 | United States | 6744 | 47 | Inquiry |
| Supplier | Advantage |
|---|---|
| City Chemical LLC | 58 |
| Chongqing Chemdad Co., Ltd | 58 |
| Shaanxi Didu New Materials Co. Ltd | 58 |
| City Chemicals Corporation | 72 |
| Aithaca Chemical Corp. | 30 |
| Pfaltz & Bauer, Inc. | 72 |
| VWR International | 82 |
| MP Biomedicals, Inc. | 81 |
| Service Chemical Inc. | 71 |
| 3B Scientific Corporation | 47 |





