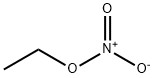
Ethyl nitrate
- CAS No.
- 625-58-1
- Chemical Name:
- Ethyl nitrate
- Synonyms
- Ethyl nitrate;1-Nitrooxyethane;Nitric acid ethyl;Nicorandil Impurity 23;Nitric acid ethyl ester;Nicorandil Impurity 13 (Ethyl Nitrate)
- CBNumber:
- CB6851985
- Molecular Formula:
- C2H5NO3
- Molecular Weight:
- 91.07
- MOL File:
- 625-58-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/11/28 16:31:43
| Melting point | -94.59°C |
|---|---|
| Boiling point | 87.25°C |
| Density | 1.1084 |
| refractive index | 1.3852 |
| solubility | 1.3 ml soluble in 100 ml water at 55°C; miscible with alcohol and ether |
| Dielectric constant | 19.7(20℃) |
| EPA Substance Registry System | Ethyl nitrate (625-58-1) |
Ethyl nitrate Chemical Properties,Uses,Production
Description
Ethyl nitrate has formula C2H5NO3. It is used in organic synthesis and as an intermediate in the preparation of some drugs, dyes, and perfumes.
Ethyl nitrate is found in the atmosphere, where it can react with other gases to form smog. Originally thought to be a pollutant, formed mainly by the combustion of fossil fuels, recent analysis of ocean water samples reveal that in places where cool water rises from the deep, the water is saturated with alkyl nitrates, likely formed by natural processes.
Chemical Properties
Ethyl nitrate is capable of explosive decomposition when exposed to heat.
Uses
Organic synthesis, drugs, perfumes, dyes, rocket propellant.
Preparation
Ethyl nitrate has been prepared by bubbling gaseous nitryl fluoride through ethanol at ?10 °C . The reaction was subsequently studied in detail.
Production Methods
The alkyl nitrates are produced from the corresponding alcohols by esterification with nitric acid in the presence of urea or urea nitrate.
General Description
A clear colorless liquid with a pleasant odor. Flash point 50°F. Prolonged exposure to fire or heat may cause vigorous decomposition and rupturing of the container. Denser than water and insoluble in water. Vapors are heavier than air. Produces toxic oxides of nitrogen during combustion.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Organonitrates, such as Ethyl nitrate, range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. Nitroalkanes are milder oxidizing agents, but still react violently with reducing agents at higher temperature and pressures. Nitroalkanes react with inorganic bases to form explosive salts. The presence of metal oxides increases the thermal sensitivity of nitroalkanes. Nitroalkanes with more than one nitro group are generally explosive.
Hazard
Flammable, dangerous, fire and explosion risk.
Health Hazard
In humans ethyl nitrate can cause headache, narcosis and vomiting, but no cases of industrial intoxication have been reported .
Industrial uses
Ethyl nitrate is used in the synthesis of certain drugs, perfumes and dyes. It has also found some use as a rocket fuel.
Safety Profile
A poison by intraperitoneal route. Mutation data reported. A very dangerous fire hazard when exposed to heat or flame; can react vigorously with oxibzing materials. A moderate explosion hazard when exposed to heat (explodes @ 185'F). To fight fire, use foam, CO2, dry chemical, water to blanket fire. Incompatible with Lewis acids. When heated to decomposition it emits toxic fumes of NOx. See also NITRATES and ESTERS.
Metabolism
There is no information concerning the in vivo metabolism of ethyl nitrate, but it seems likely that one route for biotransformation is hydrolysis to yield an alcohol and nitrate. Such a pathway is common to other alkyl nitrates such as nitroglycerin and amyl nitrate.
Ethyl nitrate Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| REDDY N REDDY PHARMACEUTICALS | +91-9848096759 +91-9848096759 | Hyderabad, India | 1989 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | 18192627656 | China | 2215 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49999 | 58 | Inquiry |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 15229059051 | China | 9980 | 58 | Inquiry |
| Guangdong Fangxin Biotechnology Co., Ltd. | 0751-2838688 13376594225 | China | 9938 | 58 | Inquiry |
| Shenzhen Excellent Biomedical Technology Co., Ltd. | 18318671572 | China | 4993 | 58 | Inquiry |
| Qiyuan (Guangdong) Pharmaceutical Chemical Co., Ltd. | 400-666-9280 18026564465 | China | 8009 | 58 | Inquiry |
| Shanghai Zerui Biotechnology Co., Ltd. | 021-54580889 18321124657 | China | 10614 | 58 | Inquiry |
| Hubei Ruinuo Pharmaceutical Technology Co., Ltd | 13165688766 | China | 5000 | 58 | Inquiry |
625-58-1(Ethyl nitrate)Related Search:
1of4
chevron_right





