FORTIMICIN
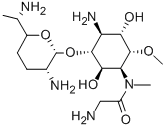
- CAS No.
- 55779-06-1
- Chemical Name:
- FORTIMICIN
- Synonyms
- kw-1070;XK-70-1;Astricin;astromicin;FORTIMYCIN;FORTIMICIN;ASTROMYCIN;fortimicina;Fortimycin A;antibiotickw-1070
- CBNumber:
- CB7116881
- Molecular Formula:
- C17H35N5O6
- Molecular Weight:
- 405.49
- MOL File:
- 55779-06-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2022/12/21 16:56:50
SAFETY
Risk and Safety Statements
| Toxicity | LD50 (of the sulfate salt) in mice (mg/kg): 380 i.v.; 400 s.c. (Nara, 1977) |
|---|
FORTIMICIN Chemical Properties,Uses,Production
Definition
ChEBI: An amino cyclitol glycoside that is L-chiro-inositol in which the hydroxy groups at positions 1, 4, and 6 are replaced by aminoacetyl)methylamino, amino, and methoxy groups, respectively, and in which the hydroxy group at posi ion 3 is converted to the corresponding 2,6-diamino-2,3,4,6,7-pentadeoxy-beta-L-lyxo-heptopyranoside. The major component of fortimicin, obtained from Micromonospora olivasterospora. It is adm nistered (as the sulfate salt) by intramuscular injection or intravenous infusion for the treatment of severe systemic infections due to sensitive Gram-negative organisms.
Antimicrobial activity
A pseudodisaccharide aminoglycoside produced by
Micromonospora olivoasterospora. Formulated as the sulfate.
Intrinsic activity is similar to that of amikacin for most
groups of organisms, but activity against Ps. aeruginosa is relatively
poor. It is resistant to many aminoglycoside-modifying
enzymes, but is sensitive to AAC(3) and the APH(2″)/AAC(6′)
bifunctional enzyme.
Peak concentrations of 10–12 mg/L were found in the
blood following 200 mg intravenous or intramuscular administration
to volunteers. The plasma half-life was 1.5–2 h. Over
85% of the drug was recovered in urine during the 8 h following
administration.
Toxicity and side effects are similar to those observed with
other aminoglycosides. Where the drug is available it is used
instead of amikacin in the treatment of infections caused by
susceptible organisms.
FORTIMICIN Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | China | 49739 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 | United States | 19973 | 58 | Inquiry |
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +86-19164747840 +86-13119157289 | China | 2969 | 58 | Inquiry |
| BOC Sciences | 1-631-485-4226; 16314854226 | United States | 14059 | 65 | Inquiry |
| Zhejiang Huida Biotech Co., LTD | 0571-0571-89903882 15990081639 | China | 3705 | 58 | Inquiry |
| Amatek Scientific Co. Ltd. | 0512-56316828 | China | 28819 | 58 | Inquiry |
| Zhejiang Huida Biotech Co., LTD | 0571-89903882 13626641628 | China | 3656 | 58 | Inquiry |
| Sequoia Research Products Ltd. | 44 7802 291086 | United Kingdom | 3115 | 69 | Inquiry |
| AK Scientific, Inc. | 650 494 8666 | United States | 6357 | 65 | Inquiry |
55779-06-1(FORTIMICIN)Related Search:
1of4
chevron_right




