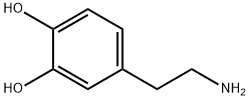
3-Hydroxytyramine
- CAS No.
- 51-61-6
- Chemical Name:
- 3-Hydroxytyramine
- Synonyms
- Dopamine;opamine;Dopamine Control;4-(2-Aminoethyl);3-Hydroxytyramine;51-61-6 3-Hydroxytyramine;3,4-Dihydroxyphenethylamine;Noradrenaline EP Impurity C;3-Hydroxytyramine USP/EP/BP;β-Hydroxytyramine (Dopamine)
- CBNumber:
- CB72130502
- Molecular Formula:
- C8H11NO2
- Molecular Weight:
- 153.18
- MOL File:
- 51-61-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/7/4 15:13:24
| Melting point | 218-220 ºC |
|---|---|
| Boiling point | 276.1°C (rough estimate) |
| Density | 1.1577 (rough estimate) |
| refractive index | 1.4770 (estimate) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | Aqueous Acid (Slightly), DMSO (Slightly, Heated), Methanol (Slightly) |
| pka | 8.9(at 25℃) |
| form | Solid |
| color | Light Brown to Brown |
| Stability | Hygroscopic |
| InChI | InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 |
| InChIKey | VYFYYTLLBUKUHU-UHFFFAOYSA-N |
| SMILES | C1(O)=CC=C(CCN)C=C1O |
| NIST Chemistry Reference | Dopamine(51-61-6) |
| EPA Substance Registry System | Dopamine (51-61-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS08,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H410-H361-H302-H400 | |||||||||
| Precautionary statements | P273-P391-P501-P273-P391-P501-P201-P202-P281-P308+P313-P405-P501-P264-P270-P301+P312-P330-P501 | |||||||||
| Hazardous Substances Data | 51-61-6(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
3-Hydroxytyramine Chemical Properties,Uses,Production
Description
Dopamine, abbreviated DA, is a biosynthetic compound and neurotransmitter produced in the body from the amino acid tyrosine by several pathways. It is synthesized in the adrenal gland where it is a precursor to other hormones (see Epinephrine) and in several portions of the brain, principally the substantia nigra and hypothalamus.
History
Dopamine is stored in vesicles in the brain’s presynaptic nerve terminals. It is closely associated with its immediate precursor, l-Dopa (levodopa). Casmir Funk (1884–1967) first synthesized Dopa in racemic form in 1911 and considered Dopa a vitamin. In 1913, Marcus Guggenheim, a biochemist from Hoff man-LaRoche, isolated l-Dopa from seedlings of Vicia faba, the Windsor bean plant native to northern Africa and southwest Asia. Guggenheim used beans from the garden of Felix Hoff man (1868–1946), the discoverer of aspirin. Guggenheim ingested a 2.5-gram dose of l-Dopa, resulting in nausea and vomiting; he also administered small dosages to animals and did not observe any signifi cant effects. This led him to believe that l-Dopa was biologically inactive. Studies commencing in 1927 reported that Dopa played a role in glucose metabolism and aff ected arterial blood pressure. Interest in dopamine accelerated in 1938 when the German physician and pharmacologist Peter Holtz (1902–1970) and co-workers discovered the enzyme l-Dopa decarboxylase and that it converted l-Dopa into dopamine in humans and animals. Research over the next two decades focused on l-Dopa’s role as a precursor to other catecholamine hormones, its vascular effects, and its role in brain chemistry.
Uses
Dopamine(3-Hydroxytyramine) is used as a drug to treat several conditions. It can be injected as a solution ofdopamine hydrochloride, such as in the drug Intropin. It is used as a stimulant to the heartmuscle to treat heart conditions; it also constricts the blood vessels, increasing systolic bloodpressure and improving blood flow through the body. Dopamine is used in renal medicationsto improve kidney function and urination. Dopamine dilates blood vessels in the kidneys,increasing the blood supply and promoting the fl ushing of wastes from the body. Dopamineis used to treat psychological disorders such as schizophrenia and paranoia.
Definition
dopamine: A catecholamine thatis a precursor in the synthesis of noradrenalineand adrenaline. It alsofunctions as a neurotransmitter inthe brain.
Biological Functions
Quantitatively, dopamine is the most important of the
biogenic amine neurotransmitters in the CNS.The three
major distinct dopaminergic systems in the mammalian
brain are categorized according to the lengths of the
neurons. There is a system comprising ultrashort neurons
within amacrine cells of the retina and periglomerular
cells in the olfactory bulb. Of the several
intermediate-length dopaminergic neuronal systems, the
best studied are neurons in the tuberobasal ventral hypothalamus
that innervate the median eminence and the
intermediate lobe of the pituitary. These neurons are
important in the regulation of various hypothalamohypophysial
functions, including prolactin release from the
anterior pituitary.The best-categorized of the dopamine
neuronal systems are the long projections from nuclei in
the substantia nigra and ventral tegmental areas to the
limbic cortex; other limbic structures, including the
amygdaloid complex and piriform cortex; and the neostriatum
(primarily the caudate and putamen). In
Parkinson’s disease, the primary biochemical feature is a
marked reduction in the concentration of dopamine in
this long projection system.
Several classes of drugs, notably the antipsychotics,
discussed in Chapter 34, interfere with dopaminergic
transmission. In general, dopamine appears to be an inhibitory
neurotransmitter. Five dopamine receptors
have been identified; the most important and best studied
are the D1- and D2-receptor groups.The D1-receptor,
which increases cyclic adenosine monophosphate
(cAMP) by activation of adenylyl cyclase, is located primarily
in the region of the putamen, nucleus accumbens,
and in the olfactory tubercle. The D2-receptor decreases
cAMP, blocks certain calcium channels, and
opens certain potassium channels.
General Description
Dopamine (Intropin) acts primarily on 1-and 1-adrenergic receptors, increasing systemic vascularresistance and exerting a positive inotropic effect on theheart. It must be administered by an intravenous route, becauseoral administration results in rapid metabolism byMAO and/or catechol-O-methyltransferase (COMT).
Mechanism of action
Dopamine is found in every sympathetic neuron and ganglion in the CNS. As a drug, and in addition to stimulation of dopaminergic receptors, dopamine indirectly stimulates both α- and β-adrenoreceptors. Dopamine also causes a release of endogenous norepinephrine. The mechanism of action is based on the excitatory effect on β-adrenoreceptors (in low and moderate doses), as well as on α-adrenoreceptors (in large doses). It has a positive inotropic effect on the heart, increases blood supply, selectively widens renal and mesenteric blood vessels, does not elevate blood pressure, and slightly increases the frequency of heartbeats.
Clinical Use
Although not strictly an adrenergic drug, dopamine is a catecholamine with properties related to the cardiovascular activities of the other agents in this chapter. Dopamine acts on specific dopamine receptors to dilate renal vessels, increasing renal blood flow. Dopamine also stimulates cardiac β1-receptors through both direct and indirect mechanisms. It is used to correct hemodynamic imbalances induced by conditions such as shock, myocardial infarction, trauma, or congestive heart failure. As a catechol and primary amine, dopamine is rapidly metabolized by COMT and MAO and, similar to dobutamine, has a short duration of action with no oral activity. It is administered as an intravenous infusion.
Environmental Fate
Dopamine quinones may irreversibly alter protein function through the formation of 5-cysteinyl-catechols on the proteins. The formation of dopamine quinone-alpha-synuclein consequently increases cytotoxic protofibrils and the covalent modification of tyrosine hydroxylase by dopamine quinones. The melaninsynthetic enzyme tyrosinase in the brain may rapidly oxidize excess amounts of cytosolic dopamine and prevent slowly progressive cell damage by auto-oxidation of dopamine, thus maintaining dopamine levels.
3-Hydroxytyramine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Aragen Life Sciences Pvt. Ltd. | +91-91-40-66929999 +91-914066929999 | Hyderabad, India | 18 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6088 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6754 | 58 | Inquiry |
| Anant Pharmaceuticals Pvt. ltd | +91 8550986868 | Maharashtra, India | 736 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | China | 3889 | 58 | Inquiry |
| Hebei Yanxi Chemical Co., Ltd. | +8618531123677 | China | 5853 | 58 | Inquiry |
Related articles
- Dopamine synthesis
- Dopamine is synthesized from phenylalanine or tyrosine via sequential reactions catalyzed mainly by PH, TH, and DOPA decarboxy....
- Jul 23,2024
- Uses and Side effects of Dopamine
- Dopamine is a neurotransmitter made in your brain. It plays a role as a “reward center” and in many body functions, including ....
- Jul 26,2022
- Synthesis and release of Dopamine
- Dopamine plays roles as both a hormone and a neurotransmitter. Dopamine inhibits prolactin release and plays a role in reward-....
- Jun 23,2022
51-61-6(3-Hydroxytyramine)Related Search:
1of4
chevron_right




