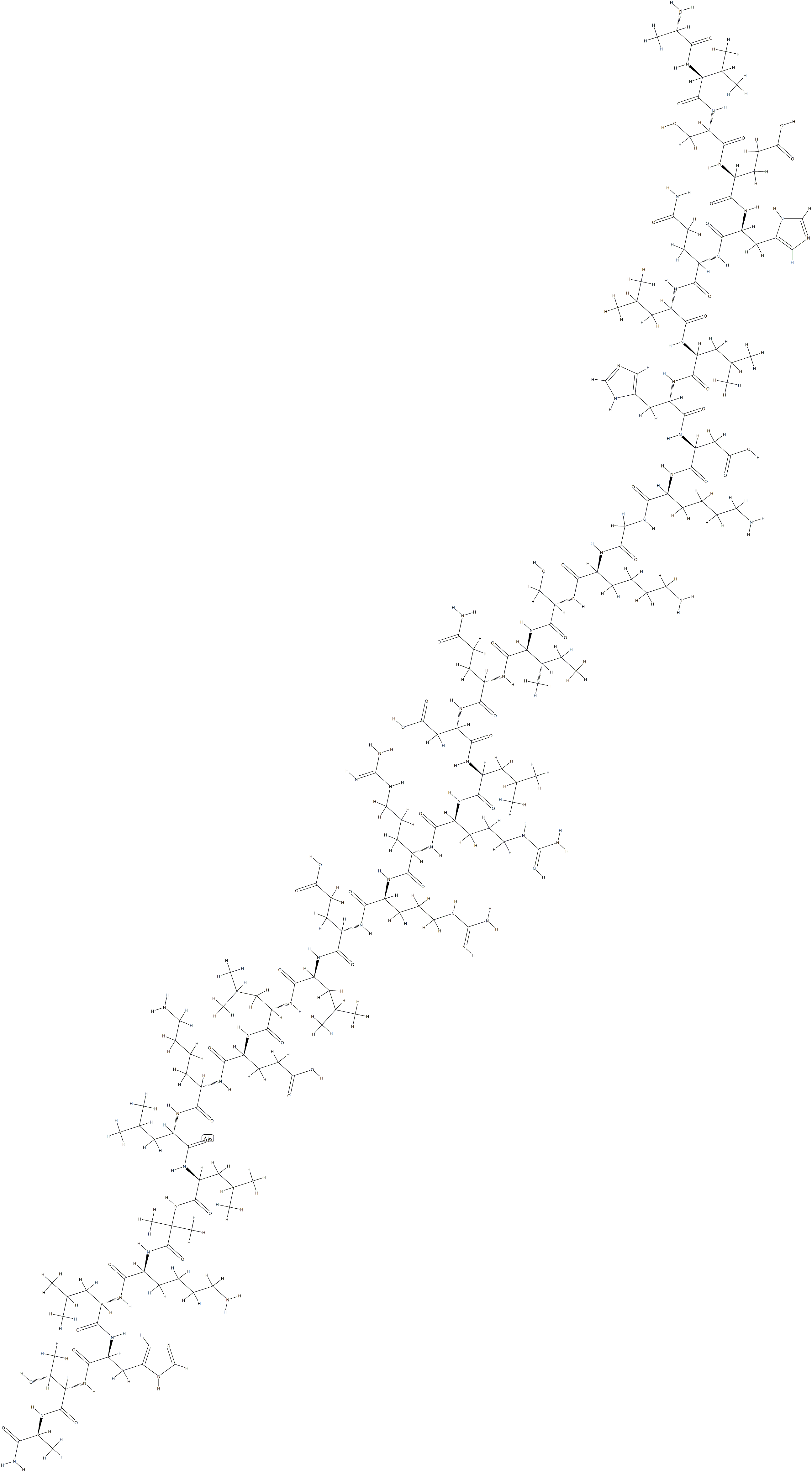
Abaloparatide
- CAS No.
- 247062-33-5
- Chemical Name:
- Abaloparatide
- Synonyms
- Abaloparatide;Abaloparatide (BA058)
- CBNumber:
- CB73166331
- Molecular Formula:
- C174H300N56O49
- Molecular Weight:
- 3960.5896
- MOL File:
- 247062-33-5.mol
- Modify Date:
- 2024/11/5 11:59:05
Abaloparatide Chemical Properties,Uses,Production
Description
Abaloparatide is a parathyroid hormone receptor 1 (PTHR1) analog and a selective PTHR1 activator. Abaloparatide enhances Gs/cAMP signaling and β-arrestin recruitment. Abaloparatide enhances bone formation and cortical structure in mice. Abaloparatide is used to treat osteoporosis.
Uses
Abaloparatide, a synthetic peptide analog of parathyroid hormone-related protein (PTHrP), has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of osteoporosis in specific patient populations. The drug is indicated for postmenopausal women who are at a high risk of fracture, which can be defined by a history of osteoporotic fracture, multiple risk factors for fracture, or those who have failed or are intolerant to other available osteoporosis therapies. In addition to its use in postmenopausal women, abaloparatide has also been approved for increasing bone density in men with osteoporosis who are at high risk for fractures. This approval extends to adult patients who have not responded effectively to or cannot tolerate other osteoporosis treatments. The drug is designed to stimulate bone formation, thereby reducing the risk of fractures associated with osteoporosis.
Mechanism of action
Abaloparatide is a human parathyroid hormone-related peptide [PTHrP(1-34)] analog, which acts as an agonist at the PTH1 receptor (PTH1R). This results in activation of the cAMP signaling pathway in target cells. In rats and monkeys, abaloparatide had an anabolic effect on bone, demonstrated by increases in BMD and bone mineral content (BMC) that correlated with increases in bone strength at vertebral and/or nonvertebral site.
Side effects
Abaloparatide is well tolerated and has mild adverse effects. Patients may experience nausea, dizziness, headache, palpitations, and hypercalciuria. Abaloparatide has been associated with supraventricular extrasystoles and orthostatic hypotension. The common (≥5%) treatment-emergent adverse drug reactions in men with osteoporosis are injection site reactions, nasopharyngitis, arthralgia, bronchitis, and hypertension.
The adverse effects reported from a randomized, double-blind, placebo-controlled trial among postmenopausal women with osteoporosis receiving 80 mcg of abaloparatide daily for 18 months include hypercalcemia (11%), dizziness (10%), nausea (8%), headache (8%), palpitations (5%), fatigue (3%), upper abdominal pain (3%), and vertigo (2%).
Abaloparatide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Apicore Pharmaceuticals Pvt Ltd | +91-2662267177 +91-2662267166 | Gujarat, India | 181 | 58 | Inquiry |
| MSN LIFE SCIENCES PRIVATE LTD | +91 84523 34200 | New Delhi, India | 104 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | China | 5978 | 58 | Inquiry |
| Zhejiang Hangyu API Co., Ltd | +8617531972939 | China | 2943 | 58 | Inquiry |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618092446649 | China | 1143 | 58 | Inquiry |
| Shanghai Yunao International Trade Co., Ltd | +8617621705551 | China | 264 | 58 | Inquiry |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | China | 10986 | 58 | Inquiry |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | China | 615 | 58 | Inquiry |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 | China | 970 | 58 | Inquiry |
247062-33-5(Abaloparatide)Related Search:
1of4
chevron_right




