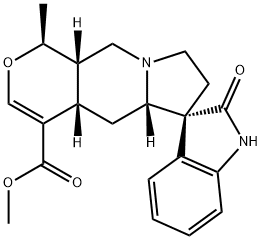
UNCARINE C
- CAS No.
- 5629-60-7
- Chemical Name:
- UNCARINE C
- Synonyms
- UNCARINE C;PTEROPODINE;Isospeciophylline;(20α)-19α-Methyl-2-oxoformosanan-16-carboxylic acid methyl ester;methyl 1-methyl-2'-oxospiro[1,4a,5,5a,7,8,10,10a-octahydropyrano[3,4-f]indolizine-6,3'-1H-indole]-4-carboxylate;methyl 1-methyl-2'-oxo-spiro[1,4a,5,5a,7,8,10,10a-octahydropyrano[3,4-f]indolizine-6,3'-1H-indole]-4-carboxylate;2'-keto-1-methyl-spiro[1,4a,5,5a,7,8,10,10a-octahydropyrano[3,4-f]indolizine-6,3'-indoline]-4-carboxylic acid methyl ester;Spiro[3H-indole-3,6'(4'aH)-[1H]pyrano[3,4-f]indolizine]-4'-carboxylic acid, 1,2,5',5'a,7',8',10',10'a-octahydro-1'-methyl-2-oxo-, methyl ester, (1'S,3R,4'aS,5'aS,10'aS)-
- CBNumber:
- CB7326387
- Molecular Formula:
- C21H24N2O4
- Molecular Weight:
- 368.43
- MOL File:
- 5629-60-7.mol
- Modify Date:
- 2023/8/24 17:20:48
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300 |
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501 |
UNCARINE C Chemical Properties,Uses,Production
Description
Isolated from Uncaria pteropoda, this alkaloid is probably stereoisomeric withMitraphylline (q.v.). It forms colourless plates when crystallized from MeOH and has [α]29DJ 9 - 102.5° (c 1.0, CHC13). The ultraviolet spectrum in MeOH has an absorption maximum at 246 mp. with a shoulder at 280 mp.. A crystalline picrate, m.p. 143-6°C; [α]D - 9.6° (c 0.5, EtOH) and a methiodide, m.p. 209-211°C after softening at 204°C; [α]D - 149° (c 0.53, EtOH) have been prepared. A solution of the alkaloid in pyridine forms an equilibrium mixture with 70 per cent isopteropodine when heated.
Uses
Uncarine C is a compound extracted from Uncaria tomentosa leaves. The compounds extracted from these leaves have exhibited immunomodulating, antiinflammatory and anti-cancer activity, attributed to the presence of tetra/pentacyclic oxindole alkaloids.
References
Yeoh, Chan, Morsingh., Tetrahedron Lett., 931 (1966)
Chan, Morsingh, Yeoh., 1. Chem. Soc., C, 2245 (1966)
Stereochemistry:
Shamma et al., 1. Amer. Chem. Soc., 89, 1739, 2799 (1967)
UNCARINE C Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | China | 1000 | 58 | Inquiry |
| Chengdu Greenpure Biopharma CO.,Ltd | +8618283602253 | China | 952 | 58 | Inquiry |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | CHINA | 1824 | 58 | Inquiry |
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 | China | 3424 | 58 | Inquiry |
| Shaanxi Pioneer Biotech Co., Ltd . | +8613259417953 | China | 3000 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| Labnetwork lnc. | +86-27-50766799 +8618062016861 | China | 19994 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 | United States | 19973 | 58 | Inquiry |
| Shanghai Moqi Biological Technology Co., LTD | 021-38218169 13341702378 | China | 9117 | 58 | Inquiry |
| Aktin Chemicals, Inc. | 028-85159085 | China | 1720 | 58 | Inquiry |
5629-60-7(UNCARINE C)Related Search:
1of4
chevron_right




