LF163892 MONOHYDRATE
- CAS No.
- 76470-66-1
- Chemical Name:
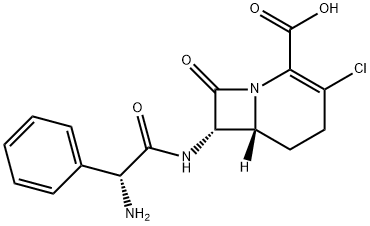
- LF163892 MONOHYDRATE
- Synonyms
- Lorabid;LOEACARBEF;LORACARBEF;LF163892 MONOHYDRATE;LORACARBEF MONOHYDRATE;LF163892 MONOHYDRATE USP/EP/BP;3-Chloro-7β-[[(R)-aminophenylacetyl]amino]-1-carbacepham-3-ene-4-carboxylic acid;(6R)-7α-[[(R)-Aminophenylacetyl]amino]-3-chloro-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;(6R,6β)-7α-[[(R)-Aminophenylacetyl]amino]-3-chloro-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;(6R,7S)-7-[[(2R)-2-AMino-2-phenylacetyl]aMino]-3-chloro-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
- CBNumber:
- CB7330045
- Molecular Formula:
- C16H16ClN3O4
- Molecular Weight:
- 349.77
- MOL File:
- 76470-66-1.mol
- Modify Date:
- 2023/4/26 16:55:04
LF163892 MONOHYDRATE Chemical Properties,Uses,Production
Description
Loracarbef is a synthetic C-5 “carba” analogue of cefaclor. The smaller methylene moiety (as compared to sulfur) would be expected to make loracarbef more reactive/potent, and this seems to be the case. It is more stable chemically, however, and this adds to its virtues.
Uses
An antibiotic, a synthetic carbacephem analogue of cefaclor, and is more stable chemically.
Definition
ChEBI: A synthetic "carba" analogue of cefaclor, with carbon replacing sulfur at position 1. Used to treat a wide range of infections caused by both gram-positive and gram-negative bacteria.
Antimicrobial activity
An oral carbacephem, with carbon replacing sulfur in the
fused ring structure. Its structure and properties are otherwise
closely related to those of cefaclor, but it has improved
chemical stability. Activity and stability to β-lactamases correspond
closely to those of cefaclor.
It is almost completely absorbed by the oral route, but
food delays absorption. A 500 mg oral dose achieves a serum
concentration of around 16 mg/L after 1.3 h. Adequate concentrations
are achieved for the treatment of upper respiratory
tract infection. Sputum concentrations have been found
to be around 2% of the corresponding plasma level. The
plasma half-life is about 1 h and protein binding is 25%.
Most of the dose is excreted unchanged in the urine, 60%
within 12 h. The elimination half-life is increased in patients
with impaired renal function. Probenecid delays excretion.
Diarrhea is the most prominent side effect, occurring in
about 4% of patients. Other gastrointestinal upsets are also
reported. It has been used for the oral treatment of upper
respiratory tract infection, skin and soft-tissue infections, and
uncomplicated urinary tract infection caused by sensitive
organisms, but is not widely available.
Clinical Use
Loracarbef (Lorabid) is the first of a series of carbacephemsprepared by total synthesis to be introduced. Carbacephemsare isosteres of the cephalosporin (or △ 3-cephem) antibioticsin which the 1-sulfur atom has been replaced by a methylene(CH2) group. Loracarbef is isosteric with cefaclor and hassimilar pharmacokinetic and microbiological properties.Thus, the antibacterial spectrum of activity resembles thatof cefaclor, but it has somewhat greater potency againstH. influenzae and M. catarrhalis, including β-lactamase–producing strains. Unlike cefaclor, which undergoes degradationin human serum, loracarbef is chemically stable inplasma. It is absorbed well orally. Oral absorption is delayedby food. The half-life in plasma is about 1 hour.
Side effects
Diarrhea is the most common adverse effect with loracarbef and, along with certain other adverse effects, is seen more frequently with children, so this lessens enthusiasm for the drug in patients younger than 12 years.
LF163892 MONOHYDRATE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29824 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 8444 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | China | 49739 | 58 | Inquiry |
| Shaanxi Xianhe Biotech Co., Ltd | +8617709210191 | China | 465 | 58 | Inquiry |
| Guangzhou PI PI Biotech Inc | 020-81716320 +86-13602409664 | China | 3635 | 55 | Inquiry |
| BOC Sciences | 1-631-485-4226; 16314854226 | United States | 14059 | 65 | Inquiry |
| MedChemExpress | 021-58955995 | United States | 6398 | 58 | Inquiry |
| Beijing Solarbio Science & Tecnology Co., Ltd. | 010-50973186 4009686088 | China | 18352 | 68 | Inquiry |
| Waterstone Technology, LLC | 317 644 0862 | United States | 6801 | 30 | Inquiry |
76470-66-1(LF163892 MONOHYDRATE)Related Search:
1of2
chevron_right




